Current Nanoscience ( IF 1.4 ) Pub Date : 2020-11-30 , DOI: 10.2174/1573413716666200313155239 Zhixiong Xie 1 , Tianyu Zhang 2 , Cheng Zhong 3
|
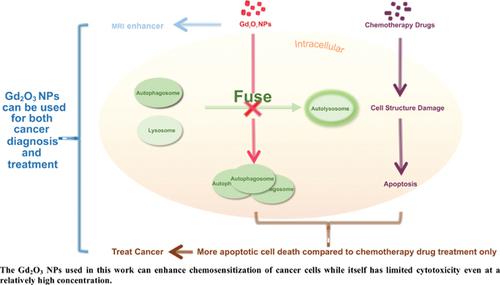
Background: During chemotherapy, drugs can damage cancer cells’ DNA and cytomembrane structure, and then induce cell death. However, autophagy can increase the chemotherapy resistance of cancer cells, reducing the effect of chemotherapy.
Objective: To block the autophagic flux in cancer cells, it is vital to enhance the anti-cancer efficacy of chemotherapy drugs; for this purpose, we test the gadolinium oxide nanoparticles (Gd2O3 NPs)’ effect on autophagy.
Methods: The cytotoxicity of Gd2O3 NPs on HeLa cells was evaluated by a (4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide assay. Then, monodasylcadaverine staining, immunofluorescence, immunoblot, and apoptosis assay were conducted to evaluate the effect of Gd2O3 NPs on autophagy and efficacy of chemotherapy drugs in human ovarian cancer cells.
Results: We found that Gd2O3 NPs, which have great potential for use as a contrast agent in magnetic resonance imaging, could block the late stage of autophagic flux in a dose-dependent manner and then cause autophagosome accumulation in HeLa cells. When co-treated with 8 μg/mL Gd2O3 NPs and 5 μg/mL cisplatin, the number of dead HeLa cells increased by about 20% compared with cisplatin alone. We observed the same phenomenon in cisplatin-resistant COC1/DDP cells.
Conclusion: We conclude that Gd2O3 NPs can block the late stage of autophagic flux and enhance the cytotoxicity of chemotherapeutic drugs in human ovarian cancer cells. Thus, the nanoparticles have significant potential for use in both diagnosis and therapy of solid tumor.
中文翻译:

氧化纳米颗粒通过阻断人卵巢癌细胞的自噬通量来增强化学治疗药物的细胞毒性。
背景:化学疗法期间,药物会破坏癌细胞的DNA和细胞膜结构,然后诱导细胞死亡。但是,自噬会增加癌细胞的化疗耐药性,降低化疗效果。
目的:阻断癌细胞自噬通量,增强化疗药物的抗癌作用至关重要。为此,我们测试了氧化oxide纳米颗粒(Gd 2 O 3 NPs)对自噬的影响。
方法:通过(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物测定评估Gd 2 O 3 NPs对HeLa细胞的细胞毒性。然后,进行了单水杨尸胺染色,免疫荧光,免疫印迹和凋亡分析,以评估Gd 2 O 3 NPs对人卵巢癌细胞自噬和化疗药物功效的影响。
结果:我们发现,Gd 2 O 3 NPs在磁共振成像中有作为造影剂的巨大潜力,它可以剂量依赖的方式阻断自噬通量的晚期,然后引起HeLa细胞中自噬体的积累。当与8μg/ mL Gd 2 O 3 NP和5μg/ mL顺铂共同处理时,与单独的顺铂相比,死亡的HeLa细胞数量增加了约20%。我们在耐顺铂的COC1 / DDP细胞中观察到相同的现象。
结论:我们得出结论,Gd 2 O 3 NPs可以阻断自噬通量的晚期并增强化学治疗药物对人卵巢癌细胞的细胞毒性。因此,纳米颗粒具有用于实体瘤的诊断和治疗的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号