Current Nanoscience ( IF 1.4 ) Pub Date : 2020-11-30 , DOI: 10.2174/1573413716666200319130830 Arehalli Manjappa 1 , Popat Kumbhar 2 , John Disouza 2 , Abhijeet D. Shete 3
|
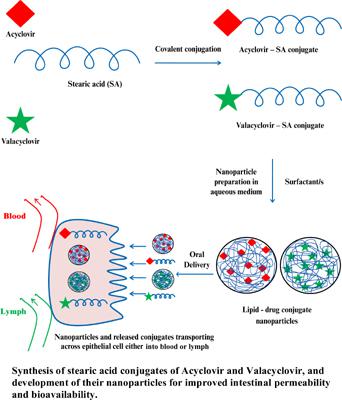
Background: The lipid-drug conjugate nanoparticles (LDC NPs), amongst other lipidbased nanoparticles, are the most accepted ones for the oral delivery of both hydrophilic and hydrophobic drugs with poor bioavailability. Besides, the LDC NPs show altered physicochemical properties of the drug and have the potential applications in targeting the drug to a specific organ.
Objective: To synthesize hydrophilic Valacyclovir (VACV)-stearic acid (SA) and lipophilic Acyclovir (ACV)-stearic acid conjugates (VACV-SAC and ACV-SAC), and develop their nanoparticles (VACV-LDC-NPs and ACV-LDC-NPs) for improved intestinal permeability.
Methods: Both VACV-SAC and ACV-SAC were synthesized and confirmed using FTIR, NMR, and DSC techniques and characterized for assay. The lipid drug conjugate nanoparticles (LDC NPs) were prepared using cold high-pressure homogenization technique and characterized for drug content, mean particle size, zeta potential, ex vivo gut permeability using rat gut sac model, and Caco-2 cell permeability.
Results: The FTIR, NMR, and DSC results confirmed the successful synthesis of LDCs. The assay of VACV-SAC and ACV-SAC was found to be 51.48±5.6% and 41.2±6.2%, respectively. The VACV-LDC-NPs and ACV-LDC-NPs showed %EE of 99.10±6.71% and 86.84±5.32%, the mean particle size of 338.7±8nm and 251.3±7nm and zeta potential of -10.8±2.31mV and -11.2±3.52mV respectively. About 91±5.2% of VACV and 84±6.5% of ACV were found permeated across the rat intestine after 480 minutes from their respective NPs. Furthermore, VACV-LDC-NPs and ACVLDC- NPs displayed a significantly higher permeability coefficient (61.5×10-6 and 59.8×10-6 cm/s, respectively) than their plain solutions.
Conclusion: The obtained remarkable permeability characteristics indicate developed LDC NPs are the potential, promising and translational approaches for effective oral delivery of poorly bioavailable hydrophilic and lipophilic drugs. Furthermore, this approach may result in moderately to significantly enhanced oral bioavailability of hydrophilic drugs as the conjugation results in amphiphilic molecules, which are further absorbed through different mechanisms across the intestinal mucosa (mainly through passive diffusion mechanism).
中文翻译:

亲水和亲脂性药物的脂质-药物缀合纳米颗粒的发展:体外肠道和Caco-2细胞渗透性比较研究。
背景:除其他基于脂质的纳米颗粒外,脂质-药物缀合物纳米颗粒(LDC NPs)是口服和生物利用度较差的亲水性和疏水性药物最常被接受的纳米颗粒。此外,LDC NPs显示出改变的药物理化特性,并且在将药物靶向特定器官方面具有潜在的应用。
目的:合成亲水性伐昔洛韦(VACV)-硬脂酸(SA)和亲脂性阿昔洛韦(ACV)-硬脂酸共轭物(VACV-SAC和ACV-SAC),并开发其纳米颗粒(VACV-LDC-NPs和ACV-LDC- NPs)可改善肠道通透性。
方法:使用FTIR,NMR和DSC技术合成并确认VACV-SAC和ACV-SAC,并进行分析表征。使用冷高压均质技术制备脂质药物偶联物纳米颗粒(LDC NPs),并用大鼠肠囊模型表征药物含量,平均粒径,ζ电位,离体肠通透性和Caco-2细胞通透性。
结果:FTIR,NMR和DSC结果证实了LDC的成功合成。发现VACV-SAC和ACV-SAC的含量分别为51.48±5.6%和41.2±6.2%。VACV-LDC-NP和ACV-LDC-NPs的%EE分别为99.10±6.71%和86.84±5.32%,平均粒径为338.7±8nm和251.3±7nm,ζ电位为-10.8±2.31mV和-11.2分别为±3.52mV。在480分钟后,从它们各自的NPs渗透到大鼠肠内的VACV约为91±5.2%,ACV约为84±6.5%。此外,VACV-LDC-NP和ACVLDC-NP显示出比普通溶液更高的渗透系数(分别为61.5×10-6和59.8×10-6 cm / s)。
结论:获得的显着渗透性特征表明,发达的LDC NPs是有效口服生物利用度差的亲水性和亲脂性药物的潜在,有前途和转化性方法。此外,这种方法可能会导致亲水性药物的口服生物利用度适度提高到显着提高,因为结合会产生两亲分子,两亲分子会通过肠粘膜的不同机制(主要是通过被动扩散机制)进一步吸收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号