Letters in Organic Chemistry ( IF 0.7 ) Pub Date : 2020-09-30 , DOI: 10.2174/1570178617666200320104923 Mohd. Zaheen Hassan 1 , Abdulrahman Alsayari 2 , Abdullatif Bin Muhsinah 1 , Jaber Abdullah Alshehri 2 , Mohammad Y. Alfaifi 3 , Serag Eldin I. Elbehairi 4 , Mater H. Mahnashi 5
|
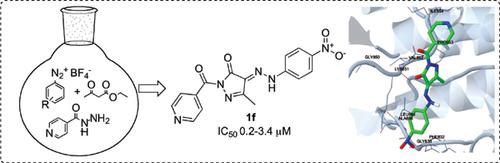
The aim of this study was to synthesize and evaluate the biological activity of pyrazole derivatives, in particular, to perform a “greener” one-pot synthesis using a solvent-free method as an alternative strategy for synthesizing hydrazono/diazenyl-pyridine-pyrazole hybrid molecules with potential anticancer activity. Effective treatment for all types of cancers is still a long way in the future due to the severe adverse drug reactions and drug resistance associated with current drugs. Therefore, there is a pressing need to develop safer and more effective anticancer agents. In this context, some hybrid analogues containing the bioactive pharmacophores viz. pyrazole, pyridine, and diazo scaffolds were synthesized by one-pot method. Herein, we describe the expedient synthesis of pyrazoles by a onepot three-component condensation of ethyl acetoacetate/acetylacetone, isoniazid, and arenediazonium salts under solvent-free conditions, and the evaluation of their cytotoxicity using a sulforhodamine B assay on three cancer cell lines. Molecular docking studies employing tyrosine kinase were also carried out to evaluate the binding mode of the pyrazole derivatives under study. 1-(4-Pyridinylcarbonyl)-3- methyl-4-(2-arylhydrazono)-2-pyrazolin-5-ones and [4-(2-aryldiazenyl)-3,5-dimethyl-1H-pyrazol-1- yl]-4-pyridinylmethanones, previously described, were prepared using an improved procedure. Among these ten products, 1-isonicotinoyl-3-methyl-4-[2-(4-nitrophenyl)hydrazono]-2-pyrazolin-5-one (1f) displayed promising anticancer activity against the MCF-7, HepG2 and HCT-116 cell lines, with an IC50 value in the range of 0.2-3.4 μM. In summary, our findings suggest that pyrazoles containing hydrazono/ diazenyl and pyridine pharmacophores constitute promising scaffolds for the development of new anticancer agents.
中文翻译:

芳基肼基/芳基重氮基吡唑类化合物:绿色一锅法无溶剂合成和抗癌评估
这项研究的目的是合成和评估吡唑衍生物的生物学活性,特别是使用无溶剂方法作为“绿色”一锅合成法,作为合成肼基/二氮烯基-吡啶-吡唑杂化物的替代策略具有潜在抗癌活性的分子。由于严重的不良药物反应和与当前药物相关的耐药性,对所有类型的癌症进行有效治疗在未来仍然很长。因此,迫切需要开发更安全和更有效的抗癌剂。在这种情况下,一些杂合类似物含有生物活性药效基团。通过一锅法合成吡唑,吡啶和重氮支架。在这里 我们描述了在无溶剂条件下通过乙酰乙酸乙酯/乙酰丙酮,异烟肼和壬二唑鎓盐的一锅式三组分缩合反应合成吡唑的方法,以及在三种癌细胞系上使用磺基罗丹明B测定法评估它们的细胞毒性。还进行了使用酪氨酸激酶的分子对接研究,以评估所研究的吡唑衍生物的结合模式。1-(4-吡啶基羰基)-3-甲基-4-(2-芳基肼基)-2-吡唑啉-5-酮和[4-(2-芳基二烯基)-3,5-二甲基-1H-吡唑-1-基使用改进的方法制备了先前描述的] -4-吡啶基甲酮。在这十种产品中,1-异烟酰酰基-3-甲基-4- [2-(4-硝基苯基)azo] -2-吡唑啉-5-酮(1f)对MCF-7,HepG2和HCT- 116个细胞系,IC50值在0.2-3.4μM范围内。总之,我们的发现表明,含有肼基/二氮烯基和吡啶药效基团的吡唑构成了开发新抗癌药的有希望的支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号