Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon supported noble metal nanoparticles as efficient catalysts for electrochemical water splitting
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0nr05659f Meng Liu 1, 2, 3, 4 , Ferdinand Hof 5, 6, 7, 8, 9 , Miriam Moro 1, 2, 3, 4 , Giovanni Valenti 1, 2, 3, 4 , Francesco Paolucci 1, 2, 3, 4 , Alain Pénicaud 5, 6, 7, 8, 9
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0nr05659f Meng Liu 1, 2, 3, 4 , Ferdinand Hof 5, 6, 7, 8, 9 , Miriam Moro 1, 2, 3, 4 , Giovanni Valenti 1, 2, 3, 4 , Francesco Paolucci 1, 2, 3, 4 , Alain Pénicaud 5, 6, 7, 8, 9
Affiliation
|
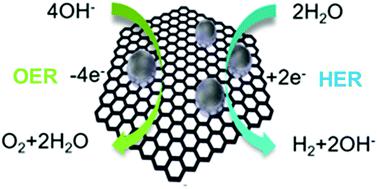
Due to an increasing requirement of clean and sustainable hydrogen energy economy, it is significant to develop new highly effective catalysts for electrochemical water splitting. In alkaline electrolyte, Platinum (Pt) shows a much slower hydrogen evolution reaction (HER) kinetics relative to acidic condition. Here, we show a versatile synthetic approach for combining different noble metals, such as Rhodium (Rh), RhPt and Pt nanoparticles, with carbon forming noble metal nanoparticles/nanocarbon composites, denoted as Rh(nP)/nC, RhPt(nP)/nC and Pt(nP)/nC, respectively. It was found that in alkaline media these composites exhibited higher performance for the HER than the commercial Pt/C. In particular, Rh(nP)/nC displayed a small overpotential of 44 mV at a current density of 5 mA cm−2 and a low Tafel slope of 50 mV dec−1. Meanwhile, it also showed a comparable activity for the oxygen evolution reaction (OER) to the benchmarking catalyst RuO2. The superior HER and OER performance benefits from the very small size of nanoparticles and synergy between carbon support and nanoparticles.
中文翻译:

碳载贵金属纳米颗粒作为电化学水分解的有效催化剂
由于对清洁和可持续的氢能源经济的日益增长的需求,开发用于电化学水分解的新型高效催化剂具有重要意义。在碱性电解液中,相对于酸性条件,铂(Pt)的氢释放反应(HER)动力学要慢得多。在这里,我们展示了一种通用的合成方法,可以将铑(Rh),RhPt和Pt纳米颗粒等不同的贵金属与碳形成贵金属纳米颗粒/纳米碳复合物(表示为Rh(nP)/ nC,RhPt(nP)/ nC和Pt(nP)/ nC分别。已发现,在碱性介质中,这些复合材料对HER的性能比市售Pt / C高。尤其是,Rh(nP)/ nC在5 mA cm -2的电流密度下显示出44 mV的小过电位且低Tafel斜率为50 mV dec -1。同时,它还显示出与基准催化剂RuO 2相当的放氧反应(OER)活性。优异的HER和OER性能得益于极小的纳米颗粒尺寸以及碳载体与纳米颗粒之间的协同作用。
更新日期:2020-10-16
中文翻译:

碳载贵金属纳米颗粒作为电化学水分解的有效催化剂
由于对清洁和可持续的氢能源经济的日益增长的需求,开发用于电化学水分解的新型高效催化剂具有重要意义。在碱性电解液中,相对于酸性条件,铂(Pt)的氢释放反应(HER)动力学要慢得多。在这里,我们展示了一种通用的合成方法,可以将铑(Rh),RhPt和Pt纳米颗粒等不同的贵金属与碳形成贵金属纳米颗粒/纳米碳复合物(表示为Rh(nP)/ nC,RhPt(nP)/ nC和Pt(nP)/ nC分别。已发现,在碱性介质中,这些复合材料对HER的性能比市售Pt / C高。尤其是,Rh(nP)/ nC在5 mA cm -2的电流密度下显示出44 mV的小过电位且低Tafel斜率为50 mV dec -1。同时,它还显示出与基准催化剂RuO 2相当的放氧反应(OER)活性。优异的HER和OER性能得益于极小的纳米颗粒尺寸以及碳载体与纳米颗粒之间的协同作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号