Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly purified extracellular vesicles from human cardiomyocytes demonstrate preferential uptake by human endothelial cells.
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0nr04278a Limor Zwi-Dantsis 1 , Charles W Winter , Ulrike Kauscher , Arianna Ferrini , Brian Wang , Thomas E Whittaker , Steve R Hood , Cesare M Terracciano , Molly M Stevens
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0nr04278a Limor Zwi-Dantsis 1 , Charles W Winter , Ulrike Kauscher , Arianna Ferrini , Brian Wang , Thomas E Whittaker , Steve R Hood , Cesare M Terracciano , Molly M Stevens
Affiliation
|
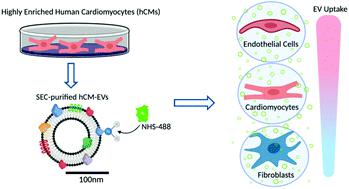
Extracellular vesicles (EVs) represent a promising cell-free alternative for treatment of cardiovascular diseases. Nevertheless, the lack of standardised and reproducible isolation methods capable of recovering pure, intact EVs presents a significant obstacle. Additionally, there is significant interest in investigating the interactions of EVs with different cardiac cell types. Here we established a robust technique for the production and isolation of EVs harvested from an enriched (>97% purity) population of human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (CMs) with size exclusion chromatography. Utilizing an advanced fluorescence labelling strategy, we then investigated the interplay of the CM-EVs with the three major cellular components of the myocardium (fibroblasts, cardiomyocytes and endothelial cells) and identified that cardiac endothelial cells show preferential uptake of these EVs. Overall, our findings provide a great opportunity to overcome the translational hurdles associated with the isolation of intact, non-aggregated human iPSC-CM EVs at high purity. Furthermore, understanding in detail the interaction of the secreted EVs with their surrounding cells in the heart may open promising new avenues in the field of EV engineering for targeted delivery in cardiac regeneration.
中文翻译:

来自人心肌细胞的高度纯化的细胞外囊泡表现出被人内皮细胞优先摄取。
细胞外囊泡(EV)是治疗心血管疾病的一种有前途的无细胞替代品。然而,缺乏能够恢复纯净、完整的 EV 的标准化和可重复的分离方法是一个重大障碍。此外,研究 EV 与不同心脏细胞类型的相互作用也引起了人们的极大兴趣。在这里,我们建立了一种强大的技术,用于通过尺寸排阻色谱法从富集(> 97% 纯度)的人诱导多能干细胞 (iPSC) 衍生的心肌细胞 (CM) 群体中生产和分离 EV。然后,我们利用先进的荧光标记策略,研究了 CM-EV 与心肌的三种主要细胞成分(成纤维细胞、心肌细胞和内皮细胞)的相互作用,并发现心脏内皮细胞优先摄取这些 EV。总的来说,我们的研究结果提供了一个很好的机会来克服与高纯度完整、非聚集的人类 iPSC-CM EV 分离相关的转化障碍。此外,详细了解分泌的 EV 与其周围心脏细胞的相互作用可能会为 EV 工程领域的心脏再生靶向递送开辟有希望的新途径。
更新日期:2020-10-08
中文翻译:

来自人心肌细胞的高度纯化的细胞外囊泡表现出被人内皮细胞优先摄取。
细胞外囊泡(EV)是治疗心血管疾病的一种有前途的无细胞替代品。然而,缺乏能够恢复纯净、完整的 EV 的标准化和可重复的分离方法是一个重大障碍。此外,研究 EV 与不同心脏细胞类型的相互作用也引起了人们的极大兴趣。在这里,我们建立了一种强大的技术,用于通过尺寸排阻色谱法从富集(> 97% 纯度)的人诱导多能干细胞 (iPSC) 衍生的心肌细胞 (CM) 群体中生产和分离 EV。然后,我们利用先进的荧光标记策略,研究了 CM-EV 与心肌的三种主要细胞成分(成纤维细胞、心肌细胞和内皮细胞)的相互作用,并发现心脏内皮细胞优先摄取这些 EV。总的来说,我们的研究结果提供了一个很好的机会来克服与高纯度完整、非聚集的人类 iPSC-CM EV 分离相关的转化障碍。此外,详细了解分泌的 EV 与其周围心脏细胞的相互作用可能会为 EV 工程领域的心脏再生靶向递送开辟有希望的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号