当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accelerated alkaline activation of peroxydisulfate by reduced rubidium tungstate nanorods for enhanced degradation of bisphenol A
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0en00840k Jian Hu 1, 2, 3, 4 , Xiangkang Zeng 1, 2, 3, 4 , Yichun Yin 1, 2, 3, 4 , Yue Liu 1, 2, 3, 4 , Yang Li 1, 2, 3, 4 , Xiaoyi Hu 1, 2, 3, 4 , Lian Zhang 1, 2, 3, 4 , Xiwang Zhang 1, 2, 3, 4
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0en00840k Jian Hu 1, 2, 3, 4 , Xiangkang Zeng 1, 2, 3, 4 , Yichun Yin 1, 2, 3, 4 , Yue Liu 1, 2, 3, 4 , Yang Li 1, 2, 3, 4 , Xiaoyi Hu 1, 2, 3, 4 , Lian Zhang 1, 2, 3, 4 , Xiwang Zhang 1, 2, 3, 4
Affiliation
|
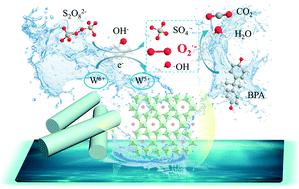
Persulfate-based advanced oxidation processes have demonstrated great prospects in engineering applications for environmental remediation. However, most of these processes are only effective under neutral or acidic conditions, and there are still very few studies on the alkaline activation of persulfate for the removal of contaminants. In this study, reduced rubidium tungstate (rRT) nanorods were synthesised and employed to activate peroxydisulfate (PDS) under alkaline conditions to effectively remove bisphenol A (BPA), a crucial pollutant in aqueous systems. It is confirmed that the rRT nanorods exhibited a superior BPA degradation rate (0.0173 min−1, 150 min) at the pH value of 11.0 compared to pH 7.0 (0.0009 min−1, 150 min) and pH 3.0 (0.0004 min−1, 150 min). The quenching test and chemical probes further unveiled that the superoxide (O2˙−) radical rather than sulfate radical (SO4˙−) and hydroxyl radical (˙OH) acted as the primary reactive oxygen species (ROSs); it was also preferentially formed under alkaline conditions. This is mainly because the electron transfer efficiency was remarkably enhanced between rRT nanorods and PDS in the alkaline rRT/PDS system, as supported by the electrochemical characterizations and X-ray photoelectron spectroscopy (XPS) analysis. This study provides new insights into the development of alkaline persulfate-activation catalysts for industrial wastewater treatment.
中文翻译:

还原钨酸钨纳米棒加速过氧二硫酸盐的碱活化,以促进双酚A的降解
基于过硫酸盐的高级氧化工艺在环境修复的工程应用中显示出了广阔的前景。然而,大多数这些方法仅在中性或酸性条件下才有效,并且关于过硫酸盐的碱性活化以去除污染物的研究还很少。在这项研究中,还原的钨酸钡(rRT)纳米棒被合成并用于在碱性条件下活化过氧二硫酸盐(PDS),以有效去除水性系统中的关键污染物双酚A(BPA)。据证实,在RRT纳米棒表现出优异的BPA退化率(0.0173分钟-1相比pH值7.0(0.0009分钟,11.0的pH值,150分钟)-1,150分钟)和pH 3.0(0.0004分钟-1,150分钟)。淬火试验和化学探针进一步公布了超氧(O 2 ˙ - )基团,而不是硫酸根(SO 4 ˙ - )和羟基自由基(OH)担任主活性氧簇(ROSS); 它也优选在碱性条件下形成。这主要是由于电化学表征和X射线光电子能谱(XPS)分析的支持,碱性rRT / PDS系统中rRT纳米棒和PDS之间的电子转移效率显着提高。这项研究为工业废水处理的碱性过硫酸盐活化催化剂的开发提供了新的见解。
更新日期:2020-11-03
中文翻译:

还原钨酸钨纳米棒加速过氧二硫酸盐的碱活化,以促进双酚A的降解
基于过硫酸盐的高级氧化工艺在环境修复的工程应用中显示出了广阔的前景。然而,大多数这些方法仅在中性或酸性条件下才有效,并且关于过硫酸盐的碱性活化以去除污染物的研究还很少。在这项研究中,还原的钨酸钡(rRT)纳米棒被合成并用于在碱性条件下活化过氧二硫酸盐(PDS),以有效去除水性系统中的关键污染物双酚A(BPA)。据证实,在RRT纳米棒表现出优异的BPA退化率(0.0173分钟-1相比pH值7.0(0.0009分钟,11.0的pH值,150分钟)-1,150分钟)和pH 3.0(0.0004分钟-1,150分钟)。淬火试验和化学探针进一步公布了超氧(O 2 ˙ - )基团,而不是硫酸根(SO 4 ˙ - )和羟基自由基(OH)担任主活性氧簇(ROSS); 它也优选在碱性条件下形成。这主要是由于电化学表征和X射线光电子能谱(XPS)分析的支持,碱性rRT / PDS系统中rRT纳米棒和PDS之间的电子转移效率显着提高。这项研究为工业废水处理的碱性过硫酸盐活化催化剂的开发提供了新的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号