当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxoiron(V) mediated selective electrochemical oxygenation of unactivated C–H and CC bonds using water as the oxygen source
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0sc03616a Bittu Chandra 1, 2, 3 , Hellan K. M. 1, 2, 3 , Santanu Pattanayak 1, 2, 3 , Sayam Sen Gupta 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-24 , DOI: 10.1039/d0sc03616a Bittu Chandra 1, 2, 3 , Hellan K. M. 1, 2, 3 , Santanu Pattanayak 1, 2, 3 , Sayam Sen Gupta 1, 2, 3
Affiliation
|
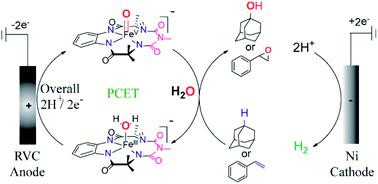
An efficient electrochemical method for the selective oxidation of C–H bonds of unactivated alkanes (BDE ≤97 kcal mol−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of alkenes using a biomimetic iron complex, [(bTAML)FeIII-OH2]−, as the redox mediator in an undivided electrochemical cell with inexpensive carbon and nickel electrodes is reported. The O-atom of water remains the source of O-incorporation in the product formed after oxidation. The products formed upon oxidation of C–H bonds display very high regioselectivity (75 : 1, 3° : 2° for adamantane) and stereo-retention (RC ∼99% for cyclohexane derivatives). The substrate scope includes natural products such as cedryl acetate and ambroxide. For alkenes, epoxides were obtained as the sole product. Mechanistic studies show the involvement of a high-valent oxoiron(V) species, [(bTAML)FeV(O)]− formed via PCET (overall 2H+/2e−) from [(bTAML)FeIII-OH2]− in CPE at 0.80 V (vs. Ag/AgNO3). Moreover, electrokinetic studies for the oxidation of C–H bonds indicate a second-order reaction with the C–H abstraction by oxoiron(V) being the rate-determining step.
C bonds of alkenes using a biomimetic iron complex, [(bTAML)FeIII-OH2]−, as the redox mediator in an undivided electrochemical cell with inexpensive carbon and nickel electrodes is reported. The O-atom of water remains the source of O-incorporation in the product formed after oxidation. The products formed upon oxidation of C–H bonds display very high regioselectivity (75 : 1, 3° : 2° for adamantane) and stereo-retention (RC ∼99% for cyclohexane derivatives). The substrate scope includes natural products such as cedryl acetate and ambroxide. For alkenes, epoxides were obtained as the sole product. Mechanistic studies show the involvement of a high-valent oxoiron(V) species, [(bTAML)FeV(O)]− formed via PCET (overall 2H+/2e−) from [(bTAML)FeIII-OH2]− in CPE at 0.80 V (vs. Ag/AgNO3). Moreover, electrokinetic studies for the oxidation of C–H bonds indicate a second-order reaction with the C–H abstraction by oxoiron(V) being the rate-determining step.
中文翻译:

Oxoiron(V)以水为氧气源,介导未活化的C–H和CC键的选择性电化学氧化
使用仿生铁络合物[(bTAML)Fe III -OH 2 ] -选择性氧化未活化烷烃(BDE≤97kcal mol -1)的C–H键和![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 烯烃的C C键的有效电化学方法据报道,作为具有廉价碳和镍电极的不分隔电化学电池中的氧化还原介体。水的O原子仍然是氧化后形成的产品中O掺入的来源。由C–H键氧化形成的产物具有很高的区域选择性(金刚烷为75:1、3°:2°)和立体保留(对于环己烷衍生物,RC约为99%)。底物的范围包括天然产物,例如乙酸双乙酰丁酯和过氧化氢。对于烯烃,环氧化物是唯一的产物。机理研究显示出高的价oxoiron的参与(V)物种,[(bTAML)的Fe V(O)] -形成经由PCET(总2H + / 2E - )从[(bTAML)铁III -OH 2 ] -在CPE中于0.80 V(相对于Ag / AgNO 3)。此外,对C–H键氧化的电动学研究表明,由氧代铁( V)提取C–H的二级反应是决定速率的步骤。
烯烃的C C键的有效电化学方法据报道,作为具有廉价碳和镍电极的不分隔电化学电池中的氧化还原介体。水的O原子仍然是氧化后形成的产品中O掺入的来源。由C–H键氧化形成的产物具有很高的区域选择性(金刚烷为75:1、3°:2°)和立体保留(对于环己烷衍生物,RC约为99%)。底物的范围包括天然产物,例如乙酸双乙酰丁酯和过氧化氢。对于烯烃,环氧化物是唯一的产物。机理研究显示出高的价oxoiron的参与(V)物种,[(bTAML)的Fe V(O)] -形成经由PCET(总2H + / 2E - )从[(bTAML)铁III -OH 2 ] -在CPE中于0.80 V(相对于Ag / AgNO 3)。此外,对C–H键氧化的电动学研究表明,由氧代铁( V)提取C–H的二级反应是决定速率的步骤。
更新日期:2020-10-14
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of alkenes using a biomimetic iron complex, [(bTAML)FeIII-OH2]−, as the redox mediator in an undivided electrochemical cell with inexpensive carbon and nickel electrodes is reported. The O-atom of water remains the source of O-incorporation in the product formed after oxidation. The products formed upon oxidation of C–H bonds display very high regioselectivity (75 : 1, 3° : 2° for adamantane) and stereo-retention (RC ∼99% for cyclohexane derivatives). The substrate scope includes natural products such as cedryl acetate and ambroxide. For alkenes, epoxides were obtained as the sole product. Mechanistic studies show the involvement of a high-valent oxoiron(V) species, [(bTAML)FeV(O)]− formed via PCET (overall 2H+/2e−) from [(bTAML)FeIII-OH2]− in CPE at 0.80 V (vs. Ag/AgNO3). Moreover, electrokinetic studies for the oxidation of C–H bonds indicate a second-order reaction with the C–H abstraction by oxoiron(V) being the rate-determining step.
C bonds of alkenes using a biomimetic iron complex, [(bTAML)FeIII-OH2]−, as the redox mediator in an undivided electrochemical cell with inexpensive carbon and nickel electrodes is reported. The O-atom of water remains the source of O-incorporation in the product formed after oxidation. The products formed upon oxidation of C–H bonds display very high regioselectivity (75 : 1, 3° : 2° for adamantane) and stereo-retention (RC ∼99% for cyclohexane derivatives). The substrate scope includes natural products such as cedryl acetate and ambroxide. For alkenes, epoxides were obtained as the sole product. Mechanistic studies show the involvement of a high-valent oxoiron(V) species, [(bTAML)FeV(O)]− formed via PCET (overall 2H+/2e−) from [(bTAML)FeIII-OH2]− in CPE at 0.80 V (vs. Ag/AgNO3). Moreover, electrokinetic studies for the oxidation of C–H bonds indicate a second-order reaction with the C–H abstraction by oxoiron(V) being the rate-determining step.
中文翻译:

Oxoiron(V)以水为氧气源,介导未活化的C–H和CC键的选择性电化学氧化
使用仿生铁络合物[(bTAML)Fe III -OH 2 ] -选择性氧化未活化烷烃(BDE≤97kcal mol -1)的C–H键和
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 烯烃的C C键的有效电化学方法据报道,作为具有廉价碳和镍电极的不分隔电化学电池中的氧化还原介体。水的O原子仍然是氧化后形成的产品中O掺入的来源。由C–H键氧化形成的产物具有很高的区域选择性(金刚烷为75:1、3°:2°)和立体保留(对于环己烷衍生物,RC约为99%)。底物的范围包括天然产物,例如乙酸双乙酰丁酯和过氧化氢。对于烯烃,环氧化物是唯一的产物。机理研究显示出高的价oxoiron的参与(V)物种,[(bTAML)的Fe V(O)] -形成经由PCET(总2H + / 2E - )从[(bTAML)铁III -OH 2 ] -在CPE中于0.80 V(相对于Ag / AgNO 3)。此外,对C–H键氧化的电动学研究表明,由氧代铁( V)提取C–H的二级反应是决定速率的步骤。
烯烃的C C键的有效电化学方法据报道,作为具有廉价碳和镍电极的不分隔电化学电池中的氧化还原介体。水的O原子仍然是氧化后形成的产品中O掺入的来源。由C–H键氧化形成的产物具有很高的区域选择性(金刚烷为75:1、3°:2°)和立体保留(对于环己烷衍生物,RC约为99%)。底物的范围包括天然产物,例如乙酸双乙酰丁酯和过氧化氢。对于烯烃,环氧化物是唯一的产物。机理研究显示出高的价oxoiron的参与(V)物种,[(bTAML)的Fe V(O)] -形成经由PCET(总2H + / 2E - )从[(bTAML)铁III -OH 2 ] -在CPE中于0.80 V(相对于Ag / AgNO 3)。此外,对C–H键氧化的电动学研究表明,由氧代铁( V)提取C–H的二级反应是决定速率的步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号