当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia Solubility, Density, and Viscosity of Choline Chloride–Dihydric Alcohol Deep Eutectic Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-24 , DOI: 10.1021/acs.jced.0c00386 Xiaoxia Deng 1 , Xiuzhi Duan 1 , Lei Gong 1 , Dongshun Deng 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-24 , DOI: 10.1021/acs.jced.0c00386 Xiaoxia Deng 1 , Xiuzhi Duan 1 , Lei Gong 1 , Dongshun Deng 1
Affiliation
|
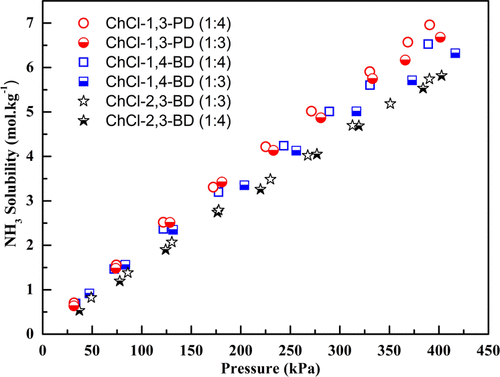
In the present work, dihydric alcohols (including 1,4-butanediol, 2,3-butanediol, and 1,3-propanediol) were paired with choline chloride (ChCl) to form deep eutectic solvents (DESs) with the molar ratio of ChCl to dihydric alcohol fixed at 1:3 and 1:4. The solubility of NH3 in these DESs was systematically determined at 303.15, 313.15, 323.15, and 333.15 K under pressures up to 420.0 kPa using the isochoric saturation method and was correlated using the Krichevsky–Kasarnovsky (K–K) equation. The dissolution Gibbs free energy, enthalpy, and entropy changes of NH3 solvation were further calculated according to the temperature dependence of Henry’s constants. Based on the reported CO2 solubility data, the selectivities of NH3/CO2 in DESs used herein were calculated. The density and viscosity data of the DESs were also measured and fitted at a temperature range of 303.15–343.15 K. Overall, the dihydric alcohol-based DESs present quite promising NH3 absorption and separation performance, with a value of 3.0 mol NH3 per kg DES and an NH3/CO2 selectivity of 98 at 303.15 K and 100.0 kPa.
中文翻译:

氯化胆碱-二元醇深度共晶溶剂的氨溶解度,密度和粘度
在本工作中,将二元醇(包括1,4-丁二醇,2,3-丁二醇和1,3-丙二醇)与氯化胆碱(ChCl)配对以形成摩尔比为ChCl的深共晶溶剂(DES)。固定为1:3和1:4的二元醇。使用等容饱和法,在压力高达420.0 kPa的条件下,系统确定了NH 3在这些DES中的溶解度,分别为303.15、313.15、323.15和333.15 K,并使用Krichevsky-Kasarnovsky(K-K)方程进行了关联。根据Henry常数对温度的依赖性,进一步计算了NH 3溶剂化的吉布斯自由能的溶解,焓和熵的变化。根据所报告的CO 2溶解度数据,可以确定NH 3 / CO的选择性计算了本文使用的DES中的2。也测定了DESS的密度和粘度数据并装配在一个温度范围内303.15-343.15 K.总体而言,二元醇系DESS本相当看好NH所述的3吸收和分离性能,以3.0摩尔的NH的值3每kg DES和303.15 K和100.0 kPa的NH 3 / CO 2选择性为98。
更新日期:2020-10-08
中文翻译:

氯化胆碱-二元醇深度共晶溶剂的氨溶解度,密度和粘度
在本工作中,将二元醇(包括1,4-丁二醇,2,3-丁二醇和1,3-丙二醇)与氯化胆碱(ChCl)配对以形成摩尔比为ChCl的深共晶溶剂(DES)。固定为1:3和1:4的二元醇。使用等容饱和法,在压力高达420.0 kPa的条件下,系统确定了NH 3在这些DES中的溶解度,分别为303.15、313.15、323.15和333.15 K,并使用Krichevsky-Kasarnovsky(K-K)方程进行了关联。根据Henry常数对温度的依赖性,进一步计算了NH 3溶剂化的吉布斯自由能的溶解,焓和熵的变化。根据所报告的CO 2溶解度数据,可以确定NH 3 / CO的选择性计算了本文使用的DES中的2。也测定了DESS的密度和粘度数据并装配在一个温度范围内303.15-343.15 K.总体而言,二元醇系DESS本相当看好NH所述的3吸收和分离性能,以3.0摩尔的NH的值3每kg DES和303.15 K和100.0 kPa的NH 3 / CO 2选择性为98。











































 京公网安备 11010802027423号
京公网安备 11010802027423号