Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Rearrangement of Stilbenes to 2,2-Diaryl-2-hydroxyacetaldehydes
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-24 , DOI: 10.1021/acsomega.0c03328 Rupali G Kalshetti 1, 2 , Chepuri V Ramana 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-24 , DOI: 10.1021/acsomega.0c03328 Rupali G Kalshetti 1, 2 , Chepuri V Ramana 1, 2
Affiliation
|
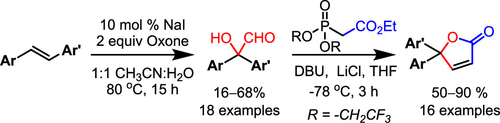
A one-pot oxone-mediated/iodine-catalyzed oxidative rearrangement of stilbenes leading to 2,2-diaryl-2-hydroxyacetaldehydes is described. Control experiments revealed that a 2,2-diarylacetaldehyde was initially formed that undergoes subsequent α-hydroxylation. The resulting α-hydroxyaldehydes have been subjected to a one-pot Still–Gennari olefination followed by cyclization, leading to 5,5-diaryl-γ-butenolides.
中文翻译:

二苯乙烯氧化重排生成 2,2-二芳基-2-羟基乙醛
描述了二苯乙烯的一锅过氧化氢介导/碘催化氧化重排,产生 2,2-二芳基-2-羟基乙醛。对照实验表明,最初形成 2,2-二芳基乙醛,随后进行 α-羟基化。所得 α-羟基醛经过一锅 Still-Gennari 烯化,然后环化,生成 5,5-二芳基-γ-丁烯内酯。
更新日期:2020-10-06
中文翻译:

二苯乙烯氧化重排生成 2,2-二芳基-2-羟基乙醛
描述了二苯乙烯的一锅过氧化氢介导/碘催化氧化重排,产生 2,2-二芳基-2-羟基乙醛。对照实验表明,最初形成 2,2-二芳基乙醛,随后进行 α-羟基化。所得 α-羟基醛经过一锅 Still-Gennari 烯化,然后环化,生成 5,5-二芳基-γ-丁烯内酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号