当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of SNAr Nucleophilic Fluorination: A Fruitful Academia-Industry Collaboration.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-24 , DOI: 10.1021/acs.accounts.0c00471 Yi Yang See 1 , María T Morales-Colón 1 , Douglas C Bland 2 , Melanie S Sanford 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-24 , DOI: 10.1021/acs.accounts.0c00471 Yi Yang See 1 , María T Morales-Colón 1 , Douglas C Bland 2 , Melanie S Sanford 1
Affiliation
|
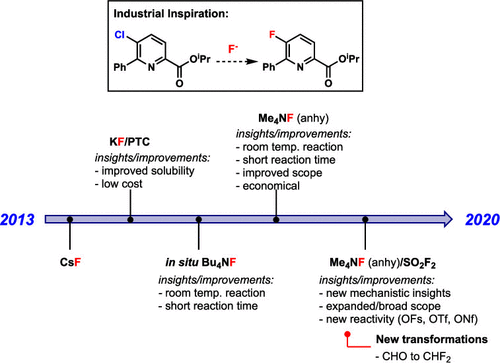
The identification of reliable, general, and high yielding methods for the formation of C(sp2)–fluorine bonds remains a major challenge for synthetic organic chemists. A very common approach involves nucleophilic aromatic fluorination (SNAr fluorination) reactions of aryl chlorides or nitroarenes. Despite being known for more than a century, traditional SNAr fluorination reactions suffer from significant limitations, particularly on a process scale. These include the high cost of common reagents [e.g., cesium fluoride (CsF)], a requirement for elevated temperatures and long reaction times, poor functional group tolerance, and the need for rigorous exclusion of water. This Account summarizes our collaboration with Corteva Agriscience (previously Dow Agrosciences) to address many of these challenges. This collaboration has provided a platform for fundamental scientific advances involving the development of new methods, reagents, and substrates for mild and high yielding nucleophilic fluorination reactions.
中文翻译:

SNAr亲核氟化的发展:产学界的卓有成效的合作。
鉴定可靠,通用和高产率的方法来形成C(sp 2)-氟键仍然是合成有机化学家的主要挑战。一个非常常见的方法包括亲核芳族氟化(S Ñ氩氟化)芳基氯化物或硝基芳烃的反应。尽管享誉一个多世纪,但传统的S NAr氟化反应受到明显的限制,特别是在工艺规模上。这些包括普通试剂的高成本[例如,氟化铯(CsF)],对高温和长反应时间的要求,对官能团的耐受性差以及对水的严格排除。此帐户总结了我们与Corteva Agriscience(以前是Dow Agrosciences)的合作,以应对许多挑战。这项合作为基础科学进步提供了一个平台,涉及开发用于轻度和高产率亲核氟化反应的新方法,试剂和底物的开发。
更新日期:2020-10-21
中文翻译:

SNAr亲核氟化的发展:产学界的卓有成效的合作。
鉴定可靠,通用和高产率的方法来形成C(sp 2)-氟键仍然是合成有机化学家的主要挑战。一个非常常见的方法包括亲核芳族氟化(S Ñ氩氟化)芳基氯化物或硝基芳烃的反应。尽管享誉一个多世纪,但传统的S NAr氟化反应受到明显的限制,特别是在工艺规模上。这些包括普通试剂的高成本[例如,氟化铯(CsF)],对高温和长反应时间的要求,对官能团的耐受性差以及对水的严格排除。此帐户总结了我们与Corteva Agriscience(以前是Dow Agrosciences)的合作,以应对许多挑战。这项合作为基础科学进步提供了一个平台,涉及开发用于轻度和高产率亲核氟化反应的新方法,试剂和底物的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号