当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Apparent Umpolung Reactivity of Indole via [Au]-Catalysed Cyclisation and Lewis-Acid Mediated Allylation.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-24 , DOI: 10.1002/chem.202003441 Mahesh H Shinde 1, 2 , Chepuri V Ramana 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-24 , DOI: 10.1002/chem.202003441 Mahesh H Shinde 1, 2 , Chepuri V Ramana 1, 2
Affiliation
|
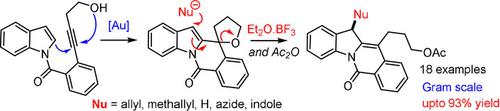
The sequential functionalization of indole C2 and C3 in an umpolung fashion was executed with a predesigned substrate and choice of reagents. The developed method comprises gold‐catalysed alkynol cycloisomerisation/intramolecular addition of C2 of indole and subsequent BF3⋅OEt2‐mediated regioselective C3 allylation, resulting in the synthesis of the functionalized indoloisoquinolinone scaffold. The reaction involves 5‐endo‐alkynol cycloisomerisation and the dearomative addition of indole C2 to the intermediate oxocarbenium cation, which results in two equilibrating fused and spiropentacyclic intermediates, which upon treatment with allyl silane in the presence of BF3⋅OEt2, undergo selective indole C3 allylation. Other nucleophiles, such as hydride, azide and indole, were also found to be compatible with this process.
中文翻译:

经由[Au]催化的环化和路易斯酸介导的烯丙基化,吲哚的表观Uppolung反应性。
用预先设计的底物和试剂的选择,将吲哚C2和C3以功能增强的顺序官能化。所提出的方法包括:金-催化的炔醇cycloisomerisation /分子内加成C2的吲哚和随后的BF的3 ⋅ OET 2介导的区域选择性C3烯丙基化,从而导致在官能indoloisoquinolinone支架的合成。该反应涉及5-内炔基环异构化和将吲哚C2脱芳香基加成到中间氧碳鎓阳离子上,这导致两个平衡的稠合和螺环戊二烯中间体,在BF 3 · OEt 2存在下用烯丙基硅烷处理,进行选择性吲哚C3烯丙基化。还发现其他亲核试剂,例如氢化物,叠氮化物和吲哚也与此过程相容。
更新日期:2020-09-24
中文翻译:

经由[Au]催化的环化和路易斯酸介导的烯丙基化,吲哚的表观Uppolung反应性。
用预先设计的底物和试剂的选择,将吲哚C2和C3以功能增强的顺序官能化。所提出的方法包括:金-催化的炔醇cycloisomerisation /分子内加成C2的吲哚和随后的BF的3 ⋅ OET 2介导的区域选择性C3烯丙基化,从而导致在官能indoloisoquinolinone支架的合成。该反应涉及5-内炔基环异构化和将吲哚C2脱芳香基加成到中间氧碳鎓阳离子上,这导致两个平衡的稠合和螺环戊二烯中间体,在BF 3 · OEt 2存在下用烯丙基硅烷处理,进行选择性吲哚C3烯丙基化。还发现其他亲核试剂,例如氢化物,叠氮化物和吲哚也与此过程相容。









































 京公网安备 11010802027423号
京公网安备 11010802027423号