当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single‐Step Glycosylations with 13C‐Labelled Sulfoxide Donors: A Low‐Temperature NMR Cartography of the Distinguishing Mechanistic Intermediates
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202003850 Andrés G Santana 1 , Laura Montalvillo-Jiménez 1 , Laura Díaz-Casado 1 , Enrique Mann 1 , Jesús Jiménez-Barbero 2, 3 , Ana M Gómez 4 , Juan Luis Asensio 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202003850 Andrés G Santana 1 , Laura Montalvillo-Jiménez 1 , Laura Díaz-Casado 1 , Enrique Mann 1 , Jesús Jiménez-Barbero 2, 3 , Ana M Gómez 4 , Juan Luis Asensio 1
Affiliation
|
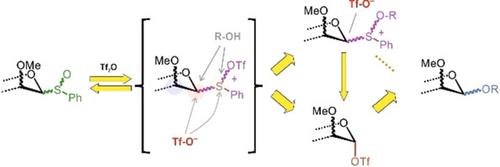
Glycosyl sulfoxides have gained recognition in the total synthesis of complex oligosaccharides and as model substrates for dissecting the mechanisms involved. Reactions of these donors are usually performed under pre‐activation conditions, but an experimentally more convenient single‐step protocol has also been reported, whereby activation is performed in the presence of the acceptor alcohol; yet, the nature and prevalence of the reaction intermediates formed in this more complex scenario have comparatively received minimal attention. Herein, a systematic NMR‐based study employing both 13C‐labelled and unlabelled glycosyl sulfoxide donors for the detection and monitoring of marginally populated intermediates is reported. The results conclusively show that glycosyl triflates play a key role in these glycosylations despite the presence of the acceptor alcohol. Importantly, the formation of covalent donor/acceptor sulfonium adducts was identified as the main competing reaction, and thus a non‐productive consumption of the acceptor that could limit the reaction yield was revealed.
中文翻译:

具有13C缩合亚砜供体的单步糖基化:区分机理中间体的低温NMR谱图
糖基亚砜在复杂寡糖的全合成中得到了认可,并作为解剖相关机理的模型底物。这些供体的反应通常在预活化条件下进行,但据报道,实验上更方便的单步操作是在受体醇存在下进行活化。然而,在这种更复杂的情况下形成的反应中间体的性质和普遍程度受到的关注相对较少。在此,一项基于NMR的系统研究同时采用了13种方法据报道,有C标记和未标记的糖基亚砜供体可用于检测和监测边缘人群的中间体。结果最终表明,尽管存在受体醇,但是糖基三氟甲磺酸酯在这些糖基化中起关键作用。重要的是,共价供体/受体sulf加合物的形成被认为是主要的竞争反应,因此揭示了受体的非生产性消耗会限制反应产率。
更新日期:2020-09-23
中文翻译:

具有13C缩合亚砜供体的单步糖基化:区分机理中间体的低温NMR谱图
糖基亚砜在复杂寡糖的全合成中得到了认可,并作为解剖相关机理的模型底物。这些供体的反应通常在预活化条件下进行,但据报道,实验上更方便的单步操作是在受体醇存在下进行活化。然而,在这种更复杂的情况下形成的反应中间体的性质和普遍程度受到的关注相对较少。在此,一项基于NMR的系统研究同时采用了13种方法据报道,有C标记和未标记的糖基亚砜供体可用于检测和监测边缘人群的中间体。结果最终表明,尽管存在受体醇,但是糖基三氟甲磺酸酯在这些糖基化中起关键作用。重要的是,共价供体/受体sulf加合物的形成被认为是主要的竞争反应,因此揭示了受体的非生产性消耗会限制反应产率。









































 京公网安备 11010802027423号
京公网安备 11010802027423号