当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural, Infrared and Magnetic properties of MgAl Fe2-O4 Compounds: Effect of the Preparation Methods and Al Substitution
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.solidstatesciences.2020.106400 I. Khishigdemberel , E. Uyanga , H. Hirazawa , B. Enkhmend , I.A. Bobrikov , D. Sangaa , T. Kiseleva
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.solidstatesciences.2020.106400 I. Khishigdemberel , E. Uyanga , H. Hirazawa , B. Enkhmend , I.A. Bobrikov , D. Sangaa , T. Kiseleva
|
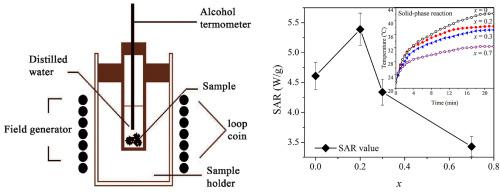
Abstract Aluminum substituted magnesium ferrite MgAlxFe2-xO4 (x = 0.0, 0.2, 0.3, 0.7 and 2.0) are prepared by two commonly-used methods: Sol-gel (SG) and Solid-phase reaction (SR). Structural studies are carried out using X-ray and neutron diffraction, and FTIR techniques, and heat generation ability in the AC magnetic field is explored by the specially designed device. Diffraction patterns revealed the formation of single cubic spinel MgAlxFe2-xO4 ferrites for SG and additional MgAl2O4 phases at higher concentration of aluminum (x > 0.3) in SR samples. Structural studies are carried out using X-ray and neutron diffraction, and FTIR techniques, scanning electron microscopy (SEM) and heat generation ability in the AC magnetic field is explored by the specially designed device. This study attempts to compare both chemical methods, and to correlate the properties with Al substitution concentration. Diffraction patterns revealed the formation of single cubic spinel MgAlxFe2-xO4 ferrites for SG and additional MgAl2O4 phases at higher concentration of aluminum (x > 0.3) in SR samples FTIR was used to confirm the spinel structure, the main vibrating modes were observed to shift to higher wave number with increasing Al3+ concentration. The tetrahedral force constant (KT) increases continuously with Al3+ concentration. The morphology investigations revealed the spherical morphology of particles with some agglomeration. The heat-generation ability was enhanced with decreasing Al concentration. Upon exposure to an external alternation magnetic field of 70 kHz and intensity of 54.3 mT, specific power absorption rate (SAR) of the Magnesium ferrite aluminates. The heat generation ability of the MgAlxFe2-xO4-SR ferrites are higher than those of MgAlxFe2-xO4-SG samples. Moreover, doping element is affected to cation distribution between tetrahedral and octahedral sites. For MgAlxFe2-xO4, heat generation ability will be influenced by additional magnetic properties and cation distribution. This will be distinguished by more detailed investigation for magnetic properties.
中文翻译:

MgAl Fe2-O4 化合物的结构、红外和磁性能:制备方法和铝取代的影响
摘要 铝取代镁铁氧体MgAlxFe2-xO4 (x = 0.0、0.2、0.3、0.7和2.0)采用溶胶-凝胶(SG)和固相反应(SR)两种常用方法制备。使用 X 射线和中子衍射以及 FTIR 技术进行结构研究,并通过专门设计的设备探索交流磁场中的发热能力。衍射图谱表明,在 SR 样品中,在较高的铝浓度 (x > 0.3) 下,SG 形成了单立方尖晶石 MgAlxFe2-xO4 铁氧体和额外的 MgAl2O4 相。使用 X 射线和中子衍射进行结构研究,并通过专门设计的设备探索 FTIR 技术、扫描电子显微镜 (SEM) 和交流磁场中的发热能力。本研究试图比较两种化学方法,并将这些特性与 Al 取代浓度相关联。衍射图谱显示,在 SR 样品中,在较高铝浓度 (x > 0.3) 下,SG 形成了单立方尖晶石 MgAlxFe2-xO4 铁氧体和额外的 MgAl2O4 相 FTIR 用于确认尖晶石结构,观察到主要振动模式转移到随着 Al3+ 浓度的增加,波数增加。四面体力常数 (KT) 随 Al3+ 浓度不断增加。形态学研究揭示了具有一些团聚的颗粒的球形形态。发热能力随着铝浓度的降低而增强。在暴露于 70 kHz 和 54.3 mT 强度的外部交变磁场时,铁氧体铝酸盐镁的比功率吸收率 (SAR)。MgAlxFe2-xO4-SR 铁氧体的发热能力高于 MgAlxFe2-xO4-SG 样品。此外,掺杂元素会影响四面体和八面体位点之间的阳离子分布。对于 MgAlxFe2-xO4,发热能力将受到附加磁性能和阳离子分布的影响。这将通过更详细的磁性研究来区分。
更新日期:2020-11-01
中文翻译:

MgAl Fe2-O4 化合物的结构、红外和磁性能:制备方法和铝取代的影响
摘要 铝取代镁铁氧体MgAlxFe2-xO4 (x = 0.0、0.2、0.3、0.7和2.0)采用溶胶-凝胶(SG)和固相反应(SR)两种常用方法制备。使用 X 射线和中子衍射以及 FTIR 技术进行结构研究,并通过专门设计的设备探索交流磁场中的发热能力。衍射图谱表明,在 SR 样品中,在较高的铝浓度 (x > 0.3) 下,SG 形成了单立方尖晶石 MgAlxFe2-xO4 铁氧体和额外的 MgAl2O4 相。使用 X 射线和中子衍射进行结构研究,并通过专门设计的设备探索 FTIR 技术、扫描电子显微镜 (SEM) 和交流磁场中的发热能力。本研究试图比较两种化学方法,并将这些特性与 Al 取代浓度相关联。衍射图谱显示,在 SR 样品中,在较高铝浓度 (x > 0.3) 下,SG 形成了单立方尖晶石 MgAlxFe2-xO4 铁氧体和额外的 MgAl2O4 相 FTIR 用于确认尖晶石结构,观察到主要振动模式转移到随着 Al3+ 浓度的增加,波数增加。四面体力常数 (KT) 随 Al3+ 浓度不断增加。形态学研究揭示了具有一些团聚的颗粒的球形形态。发热能力随着铝浓度的降低而增强。在暴露于 70 kHz 和 54.3 mT 强度的外部交变磁场时,铁氧体铝酸盐镁的比功率吸收率 (SAR)。MgAlxFe2-xO4-SR 铁氧体的发热能力高于 MgAlxFe2-xO4-SG 样品。此外,掺杂元素会影响四面体和八面体位点之间的阳离子分布。对于 MgAlxFe2-xO4,发热能力将受到附加磁性能和阳离子分布的影响。这将通过更详细的磁性研究来区分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号