Separation and Purification Technology ( IF 8.1 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.seppur.2020.117774 Yang Song , Jin Jiang , Wen Qin , Juntao Zhu , Jia Gu , Jun Ma
|
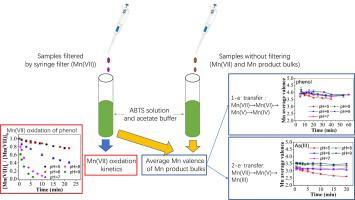
In this study, a colorimetric method using 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonate (ABTS) was adopted to investigate oxidation kinetics by permanganate (Mn(VII)) in trace levels (lower than 1 mg/L or 6 µM) and measure average manganese valence in manganese product bulks in (i) synthetic waters containing selected model compounds with electron-rich moieties, (ii) natural organic matters (NOM) isolate solution, and (iii) real water samples including river water, reservoir water and secondary wastewater effluent. The second-order rate constant for Mn(VII) oxidation of phenol determined using ABTS method was demonstrated to be comparable to that measured by traditional high-performance liquid chromatography (HPLC) method at pH 5–9. This method was applied to measure the rate constants of the reactions between Mn(VII) and model compounds with electron-rich moieties. Average manganese valence in manganese product bulks from Mn(VII) oxidation of selected model compounds was lower than 4 at acidic pH, suggesting that Mn(III) was likely to be generated during Mn(VII) oxidation. Possible mechanisms for Mn(III) formation via one/two-electron process were also proposed. Mn(VII) reaction with NOM isolate sample followed two-phase kinetics with rate constants of 3.47 × 10-5 s−1 (mg C/L)-1 and 6.94 × 10-6 s−1 (mg C/L)-1 in the initial and secondary phases at pH 8. Similarly, two-phase kinetics were also found in the consumption of Mn(VII) in real waters. Mn(VII) consumption rates in different real waters followed the order of their dissolved organic carbon levels. In contrast, chlorine decay rates were mainly based on the order of their dissolved organic nitrogen levels. Moreover, average valence in manganese products in real water samples was determined as around 4, which was mainly ascribed to the one-electron-transfer processes of Mn(VII) with NOM constituents in real water samples such as phenols and amines at ambient weak alkaline pH.
中文翻译:

同时光度法测定高锰酸盐水溶液与模型有机化合物和天然有机物反应生成的锰产品中的氧化动力学和平均锰价
在这项研究中,采用比色法使用2,2'-叠氮基双(3-乙基苯并噻唑啉)-6-磺酸盐(ABTS)来研究痕量(低于1 mg)的高锰酸盐(Mn(VII))的氧化动力学。 / L或6 µM),并测量(i)包含选定模型化合物的合成水中的锰产品散装中的平均锰价,这些化合物具有富电子部分;(ii)天然有机物(NOM)分离溶液;以及(iii)真实水样包括河水,水库水和二次废水。结果表明,使用ABTS方法测定的苯酚Mn(VII)氧化的二级速率常数与传统的高效液相色谱(HPLC)方法在pH 5–9下测得的速率常数相当。该方法用于测量Mn(VII)与具有富电子部分的模型化合物之间反应的速率常数。所选模型化合物的Mn(VII)氧化导致的锰产品主体中的平均锰价在酸性pH值下低于4,这表明Mn(III)可能在Mn(VII)氧化过程中生成。还提出了通过一/二电子过程形成Mn(III)的可能机理。Mn(VII)与NOM分离样品的反应遵循两相动力学,速率常数为3.47×10 还提出了通过一/二电子过程形成Mn(III)的可能机理。Mn(VII)与NOM分离样品的反应遵循两相动力学,速率常数为3.47×10 还提出了通过一/二电子过程形成Mn(III)的可能机理。Mn(VII)与NOM分离样品的反应遵循两相动力学,速率常数为3.47×10在pH 8的初始和次级阶段,分别为-5 s -1(mg C / L)-1和6.94×10 -6 s -1(mg C / L)-1。类似地,在实际水中Mn(VII)的消耗量。在不同的真实水中,Mn(VII)的消耗速率遵循其溶解有机碳含量的顺序。相反,氯的衰减速率主要基于其溶解的有机氮含量的顺序。此外,实际水样中锰产品的平均价被确定为4左右,这主要归因于在弱碱性环境下实际水样中的Mn(VII)与NOM成分(如酚和胺)的单电子转移过程。 pH值











































 京公网安备 11010802027423号
京公网安备 11010802027423号