Molecular and Biochemical Parasitology ( IF 1.4 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.molbiopara.2020.111320 Lucila Giordana 1 , Cristina Nowicki 1
|
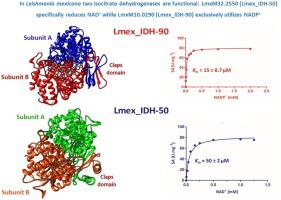
Leishmania parasites are of great relevance to public health because they are the causative agents of various long-term and health-threatening diseases in humans. Dependent on the manifestation, drugs either require difficult and lengthy administration, are toxic, expensive, not very effective or have lost efficacy due to the resistance developed by these pathogens against clinical treatments. The intermediary metabolism of Leishmania parasites is characterized by several unusual features, among which whether the Krebs cycle operates in a cyclic and/or in a non-cyclic mode is included. Our survey of the genomes of Leishmania species and monoxenous parasites such as those of the genera Crithidia and Leptomonas (http://www.tritrypdb.org) revealed that two genes encoding putative isocitrate dehydrogenases (IDHs) -with distantly related sequences- are strictly conserved among these parasites. Thus, in this study, we aimed to functionally characterize the two leishmanial IDH isoenzymes, for which we selected the genes LmxM10.0290 (Lmex_IDH-90) and LmxM32.2550 (Lmex_IDH-50) from L. mexicana. Phylogenetic analysis showed that Lmex_IDH-50 clustered with members of Subfamily I, which contains mainly archaeal and bacterial IDHs, and that Lmex_IDH-90 was a close relative of eukaryotic enzymes comprised within Subfamily II IDHs. 3-D homology modeling predicted that both IDHs exhibited the typical folding motifs recognized as canonical for prokaryotic and eukaryotic counterparts, respectively. Both IDH isoforms displayed dual subcellular localization, in the cytosol and the mitochondrion. Kinetic studies showed that Lmex_IDH-50 exclusively catalyzed the reduction of NAD+, while Lmex_IDH-90 solely used NADP+ as coenzyme. Besides, Lmex_IDH-50 differed from Lmex_IDH-90 by exhibiting a nearly 20-fold lower apparent Km value towards isocitrate (2.0 μM vs 43 μM). Our findings showed, for the first time, that the genus Leishmania differentiates not only from other trypanosomatids such as Trypanosoma cruzi and Trypanosoma brucei, but also from most living organisms, by exhibiting two functional homo-dimeric IDHs, highly specific towards NAD+ and NADP+, respectively. It is tempting to argue that any or both types of IDHs might be directly or indirectly linked to the Krebs cycle and/or to the de novo synthesis of glutamate. Our results about the biochemical and structural features of leishmanial IDHs show the relevance of deepening our knowledge of the metabolic processes in these pathogenic parasites to potentially identify new therapeutic targets.
中文翻译:

在利什曼原虫寄生虫中编码了两个系统发育上不同的异柠檬酸脱氢酶。墨西哥利什曼原虫同工酶的分子和功能表征,对NAD +和NADP +具有特异性
利什曼原虫寄生虫与公共卫生息息相关,因为它们是人类各种长期和威胁健康的疾病的病原体。根据表现,药物由于这些病原体对临床治疗的耐药性而需要困难且漫长的给药时间,有毒,昂贵,效果不佳或失去疗效。利什曼原虫寄生虫的中间代谢的特征在于几个不同寻常的特征,其中包括克雷布斯循环是否以循环和/或非循环模式运行。我们对利什曼原虫种和单寄生性寄生虫(如Crithidia和Leptomonas属)的基因组进行了调查(http://www.tritrypdb.org)揭示,在这些寄生虫中,编码保守的异柠檬酸脱氢酶(IDHs)的两个基因-具有远近相关的序列-被严格保守。因此,在这项研究中,我们旨在从功能上表征两个利什曼原虫IDH同工酶,为此我们选择了来自墨西哥乳杆菌的基因LmxM10.0290(Lmex_IDH-90)和LmxM32.2550(Lmex_IDH-50)。系统发育分析表明,Lmex_IDH-50与亚家族I的成员聚在一起,后者主要包含古细菌和细菌IDH,而Lmex_IDH-90是亚家族II IDH中包含的真核酶的近亲。3-D同源性建模预测,两个IDH均表现出典型的折叠基序,分别被认为是原核和真核对应的典型折叠模体。两种IDH亚型均在胞质溶胶和线粒体中显示出双重亚细胞定位。动力学研究表明,Lmex_IDH-50专门催化NAD +的还原,而Lmex_IDH-90仅使用NADP +作为辅酶。此外,Lmex_IDH-50与Lmex_IDH-90的不同之处在于其表观K m值降低了近20倍异柠檬酸盐的值(2.0μM对43μM)。我们的研究结果首次显示,利什曼原虫属不仅通过展示两种功能性同二聚体IDH(它们对NAD +和NADP具有高度特异性)与其他锥虫(如锥虫和布鲁氏锥虫)有所区别,而且还与大多数活生物体有所区别。+。诱人的论点是,任何一种或两种类型的IDH都可能直接或间接地与克雷布斯周期和/或从头联系在一起。合成谷氨酸。我们有关利什曼原体IDH的生化和结构特征的结果表明,加深我们对这些病原体寄生虫的代谢过程的认识具有潜在的相关性,以潜在地确定新的治疗靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号