Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.molliq.2020.114367 Subrata Nayak , Anamika Ray , Sumanta Bhattacharya
|
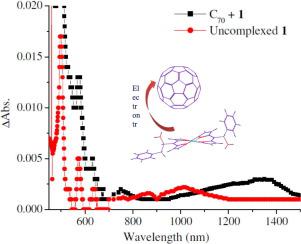
The present work reports the case study on molecular complexation of a designed porphyrin, namely, 10,20-Bis(trans-1-amino-2-phenylethyl)-porphyrin-ZnII (1) with fullerenes C60 and C70 in toluene and 1,2-dichlorobenzene (DCB). As evidenced from both UV–vis and steady state fluorescence studies, the interaction is considered to be purely non-covalent in nature. Calculation of the binding constants value (K) of the C60 (i.e., KC60-1 = 3675 dm3·mol−1 in toluene and 4050 dm3·mol−1 in DCB) and C70 complexes of 1 (i.e., KC70-1 = 18,630 dm3·mol−1 in toluene and 20,105 dm3·mol−1 in DCB) reveal that size selective non-covalent interaction prevails in present work as selectivity of binding between C70 and C60 (KC70/KC60) is estimated to be same, i.e., KC70/KC60 ~ 5.1 measured both in toluene and DCB. Steady state fluorescence measurements elicit that the quenching of fluorescence of 1 in presence of C70 in DCB occurs via both static and dynamic quenching pathway. The values of kCSs (and φCSs) for the C60-1 and C70-1 systems are evaluated to be 8.95 × 106 s−1 (and 0.037) and 2.10 × 107 s−1 (and 0.083) in DCB, respectively, which provides very good support in favour of charge separation as well as formidable quenching in case of C70-1 system compared to C60-1 system. Explicit DFT calculations in vacuo predict the geometry on orientation of bound guest (here fullerenes) with the donor (here 1) and extend very good thermodynamic support in favour of the high value of K for the C70-1 system compared to C60-1 system with the help of theoretically calculated heat of formation (ΔHf0) value of such systems, i.e., ΔHf0 (C60-1) = −2.338 kcal·mol−1 and ΔHf0 (C70-1) = −42.802 kcal·mol−1 (side-on orientation of C70).
中文翻译:

在溶液中设计的卟啉与C 60和C 70分子络合后的尺寸选择性超分子相互作用
本工作报道了一种设计的卟啉,即10,20-双(反-1-氨基-2-苯基乙基)-卟啉-Zn II(1)与富勒烯C 60和C 70在甲苯中的分子络合的案例研究。和1,2-二氯苯(DCB)。从紫外可见光谱和稳态荧光研究都可以看出,这种相互作用本质上被认为是纯非共价的。的结合常数的值(计算ķ)作为C 60(即,ķ C60- 1 = 3675分米3 ·摩尔-1的甲苯溶液和4050分米3 ·摩尔-1在DCB)和C 70的复合物1(即,ķ C70- 1 = 18630分米3 ·摩尔-1的甲苯溶液和20105分米3 ·摩尔-1在DCB)揭示在目前的工作中该尺寸选择性非共价相互作用的获胜其形式为C之间的结合选择性70和C 60(ķ C70 / ķ C60)被估计为相同的,即,ķ C70 / ķ C60 〜5.1的甲苯溶液和DCB测量两者。稳态荧光测量结果表明存在C 70时1的荧光猝灭DCB中的β-氨基丁酸通过静态和动态淬灭途径发生。的值ķ CS小号(和φ CS小号)为C 60 - 1和C 70 - 1个系统被评价为8.95×10 6 小号- 1(和0.037)和2.10×10 7 小号- 1(和0.083 )在DCB分别提供有利于电荷分离很好的支持,以及在C的情况下,淬火强大70 - 1个系统相比至C 60 - 1系统。在真空中明确DFT计算预测与捐助者(这里绑定客人(这里富勒烯)的定向几何1)和赞成高附加值的延伸很好的热力支持ķ为C 70 - 1个系统与C 60 - 1个系统与地层的理论计算的热的帮助(Δ ħ ˚F 0)这样的系统中的值,即,Δ ħ ˚F 0(C 60 - 1)= -2.338·千卡摩尔-1和Δ ħ ˚F 0(C 70 - 1)=-42.802kcal·mol -1(C 70的侧向取向)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号