Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.jmgm.2020.107761 Haiyan Wang 1 , Feng-Ling Liu 1
|
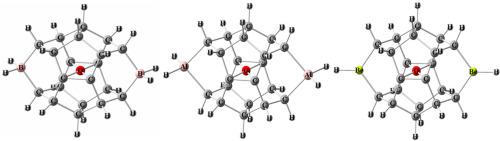
Oxygen usually forms two single bonds with other atoms. In contrast, here we reported the neutral molecules with the planar O(C)4-type tetracoordinate oxygen substructures. The molecules with planar tetracoordinate oxygen, as desirable targets for studying the nonclassical structures and unusual bonding features, remain an important challenge in only carbon-based groups as ligands. In this work, several neutral molecules with planar tetracoordinate oxygen atom surrounded by four carbon-based groups in the pagodane-like derivatives, have been designed out by using the “charge-compensation” and “mechanical” strategies, and studied at the B3LYP/6–311++G(3df,3pd) level of theory. The computational results show that they are all minima on the potential energy surfaces without any imaginary vibrational frequency, and unlike ptC delocalization of the oxygen 2pz lone pair in ptO(C)4 is no longer the principal reason for stabilizing a ptO substructure.
中文翻译:

DFT研究平面四配位氧被四个碳基团包围的中性分子
氧通常与其他原子形成两个单键。相反,在这里我们报道了具有平面O(C)4的中性分子型四配位氧亚结构。具有平面四配位氧的分子,作为研究非经典结构和异常键合特征的理想目标,在仅基于碳的基团作为配体方面仍然是一项重要挑战。在这项工作中,通过使用“电荷补偿”和“机械”策略设计出了一些具有平面四配位氧原子的中性分子,它们被包围在宝塔丹状衍生物中的四个碳基上,并在B3LYP / 6–311 ++ G(3df,3pd)的理论水平。计算结果表明,它们在势能面上都是极小值,没有任何假想的振动频率,并且与ptO(C)4中的氧2 p z孤对的ptC离域化不同 不再是稳定ptO子结构的主要原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号