Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.csbj.2020.09.028 Gemma Navarro , Angel Gonzalez , Stefano Campanacci , Rafael Rivas-Santisteban , Irene Reyes-Resina , Nil Casajuana-Martin , Arnau Cordomí , Leonardo Pardo , Rafael Franco
|
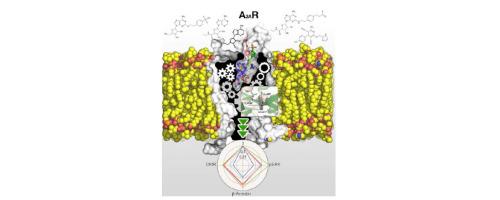
Biased agonism, the ability of agonists to differentially activate downstream signaling pathways by stabilizing specific receptor conformations, is a key issue for G protein-coupled receptor (GPCR) signaling. The C-terminal domain might influence this functional selectivity of GPCRs as it engages G proteins, GPCR kinases, β-arrestins, and several other proteins. Thus, the aim of this paper is to compare the agonist-dependent selectivity for intracellular pathways in a heterologous system expressing the full-length (A2AR) and a C-tail truncated (A2AΔ40R lacking the last 40 amino acids) adenosine A2A receptor, a GPCR that is already targeted in Parkinson’s disease using a first-in-class drug. Experimental data such as ligand binding, cAMP production, β-arrestin recruitment, pERK1/2 phosphorylation and dynamic mass redistribution assays, which correspond to different steps of the signaling pathways, were measured upon the action of structurally diverse compounds (the agonists adenosine, NECA, CGS-21680, PSB-0777 and LUF-5834 and the SCH-58261 antagonist) in cells expressing A2AR and A2AΔ40R. The results show that taking cAMP levels and the endogenous adenosine agonist as references, the main difference in bias was obtained with PSB-0777 and LUF-5834. The C-terminus is dispensable for both G-protein and β-arrestin recruitment and also for MAPK activation. Unrestrained molecular dynamics simulations, at the μs timescale, were used to understand the structural arrangements of the binding cavity, triggered by these chemically different agonists, facilitating G protein binding with different efficacy.
中文翻译:

对全长和C末端截短的腺苷A 2A受体有偏向激动作用的实验和计算分析
偏置激动剂(激动剂通过稳定特定受体构象差异激活下游信号通路的能力)是G蛋白偶联受体(GPCR)信号传导的关键问题。C末端结构域与G蛋白,GPCR激酶,β-arrestin和其他几种蛋白质结合时,可能会影响GPCR的这种功能选择性。因此,本文的目的是比较对细胞内途径的激动剂依赖性选择性在表达全长(A的异源系统2A R)和一个C-尾截断(A 2A Δ40 ř缺少最后40个氨基酸)腺苷A 2A受体,一种使用一流药物已经靶向帕金森氏病的GPCR。根据结构多样的化合物(激动剂腺苷,NECA)的作用,测量了与配体信号传导途径不同步骤相对应的实验数据,例如配体结合,cAMP产生,β-arrestin募集,pERK1 / 2磷酸化和动态质量重新分布测定等。 ,CGS-21680,PSB-0777和LUF-5834和SCH-58261拮抗剂)在表达A细胞2A R和甲2A Δ40R.结果显示,以cAMP水平和内源性腺苷激动剂为参考,使用PSB-0777和LUF-5834获得了偏差的主要差异。C末端对于G蛋白和β-arrestin募集以及MAPK激活都是必不可少的。使用μs时间尺度的无限制分子动力学模拟来了解由这些化学上不同的激动剂触发的结合腔的结构排列,从而促进具有不同功效的G蛋白结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号