Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.cej.2020.127122 Akanksha Joshi , Anuj Kumar Tomar , Gurmeet Singh , Raj Kishore Sharma
|
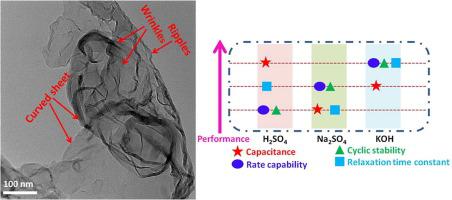
Boron is a highly pursued advance charge storage material ascribed to its lighter weight and metallic nature. However, vastly reactive bonding structure of boron is impeding its application as supercapacitive material, especially in aqueous electrolyte. Here, an energetically favourable oxidation approach is engineered to enforce the lattice distortion of β-rhombohedral phase that results in oxygen defect. This approach causes the oxidative exfoliation (few layers∼8.5 nm thickness) to maximize the electroactive surface area and also stabilizes the structure in aqueous electrolytes. These attributes combining with large conductivity (96.12 S m-1) results in an excellent cycling stability (> 80%) and rate capability (> 59%) in aqueous electrolytes. Among different pH electrolytes, oxygen defective boron nanosheet with KOH (τ∼0.83 s; 107.63 mF cm-2@2 A g-1) and H2SO4 (τ∼1.78 s; 141.55 mF cm-2@2 A g-1) shows extremely splendid pseudocapacitive charge storage. From the mechanistic study, governance of pseudocapacitive contribution is verified. Moreover, the fabricated symmetric cell (3V, BMIMBF4) with slight decline of discharge voltage (2.85 to 2.4 V) on current density increment (5 fold) exhibits a maximum specific energy of 25.1 Wh kg-1@636.13 W kg-1. This work provides a cornerstone to the upcoming studies for further advancement of highly conductive boron based capacitive electrodes.
中文翻译:

硼纳米片中用于稳定复杂键合结构的工程氧缺陷:一种高性能超级电容器的方法
硼是一种备受追捧的高级电荷存储材料,这归因于其较轻的重量和金属性质。然而,硼的反应性强的键合结构阻碍了其作为超电容材料的应用,尤其是在水性电解质中。在这里,设计了一种能量上有利的氧化方法,以强制导致氧缺陷的β-菱形面相的晶格畸变。这种方法使氧化剥落(几层厚度约8.5 nm)使电活性表面积最大化,并使水电解质的结构稳定。这些特性与大电导率(96.12 S m -1)在水性电解质中具有出色的循环稳定性(> 80%)和倍率能力(> 59%)。之间不同的pH的电解质,氧缺陷的硼纳米片与KOH(τ~0.83S; 107.63 mF及其厘米-2 @ 2 A G -1)和H 2 SO 4(τ~1.78S; 141.55 mF及其厘米-2 @ 2 A G -图1)显示了非常出色的伪电容电荷存储。通过机理研究,证实了伪电容贡献的治理。此外,在电流密度增加(5倍)时,放电电压(2.85至2.4 V)略有下降的对称电池(3V,BMIMBF4)的最大比能为25.1 Wh kg -1 @ 636.13 W kg -1。这项工作为进一步研究高导电性硼基电容电极的进一步发展奠定了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号