Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.cej.2020.127095 Ying Liang , Lijuan Feng , Xin Liu , Yuxiang Zhao , Qi Chen , Zhuyin Sui , Ning Wang
|
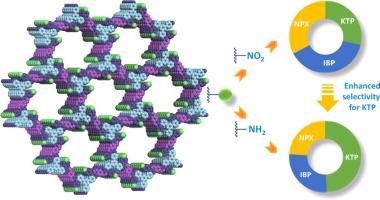
A suitable porous adsorbent with prominent adsorption performance and better selectivity for the target containments such as non-steroidal anti-inflammatory drugs (NSAIDs) is highly desired in wastewater treatment. Owing to the intrinsic attributes known as predictable structure, tunable pore size, high chemical stability as well as the reliable post-synthetic modification, covalent organic frameworks (COFs) are considered as brilliant adsorbent candidates which have drawn great attentions. Herein, two COFs with different functional groups (COF-NO2 and COF-NH2) were designed and prepared in order to enhance selective adsorption ability toward the Ketoprofen (KTP) molecule via functional group tuning. For KTP, due to amino group which could interact with not only carboxylic acid but also carbonyl group, COF-NH2 exhibited twice adsorption capacity as high as other two counterparts Ibuprofen (IBP) and Naproxen (NPX). However, there was no selectivity for those three NSAIDs when COFs containing nitro groups (COF-NO2) were used as adsorbents. We also found that the adsorption process followed the pseudo-second-order kinetic model well, which confirmed the capture of KTP onto COF-NH2 was dominated by chemical adsorption. The COF-NH2 will be a particularly promising porous adsorbent to selectively eliminate the KTP from effluents, and COFs with specific binding sites for various pollutants can be acquired via functional group tuning.
中文翻译:

通过官能团调节增强共价有机骨架对NSAID的选择性吸附
在废水处理中,非常需要对目标容器(例如非甾体类抗炎药(NSAIDs))具有突出的吸附性能和更好的选择性的合适的多孔吸附剂。由于固有的可预测结构,可调节的孔径,高化学稳定性以及可靠的合成后修饰,共价有机骨架(COFs)被认为是出色的吸附剂候选物,受到了广泛关注。在此,两个具有不同官能团的COF(COF-NO 2和COF-NH 2设计和制备)的目的是通过官能团调节来增强对酮洛芬(KTP)分子的选择性吸附能力。对于KTP,由于氨基不仅可以与羧酸发生相互作用,而且可以与羰基发生相互作用,因此COF-NH 2的吸附能力是布洛芬(IBP)和萘普生(NPX)的两倍。但是,当使用含硝基的COF(COF-NO 2)作为吸附剂时,这三种NSAID没有选择性。我们还发现吸附过程很好地遵循了伪二级动力学模型,这证实了KTP在COF-NH 2上的捕获主要是化学吸附。COF-NH 2 这将是一种特别有希望的多孔吸附剂,可以选择性地去除废水中的KTP,并且可以通过官能团调节来获得具有与各种污染物特定结合位点的COF。



























 京公网安备 11010802027423号
京公网安备 11010802027423号