Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.actbio.2020.09.033 Alexander Thomas , Isabel Orellano , Tobias Lam , Benjamin Noichl , Michel-Andreas Geiger , Anna-Klara Amler , Anna-Elisabeth Kreuder , Christopher Palmer , Georg Duda , Roland Lauster , Lutz Kloke
|
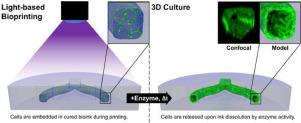
Introduction of cavities and channels into 3D bioprinted constructs is a prerequisite for recreating physiological tissue architectures and integrating vasculature. Projection-based stereolithography inherently offers high printing speed with high spatial resolution, but so far has been incapable of fabricating complex native tissue architectures with cellular and biomaterial diversity. The use of sacrificial photoinks, i.e. photopolymerisable biomaterials that can be removed after printing, theoretically allows for the creation of any construct geometry via a multi-material printing process. However, the realisation of this strategy has been challenging because of difficult technical implementation and a lack of performant biomaterials. In this work, we use our projection-based, multi-material stereolithographic bioprinter and an enzymatically degradable sacrificial photoink to overcome the current hurdles. Multiple, hyaluronic acid-based photoinks were screened for printability, mechanical properties and digestibility through hyaluronidase. A formulation meeting all major requirements, i.e. desirable printing properties, generation of sufficiently strong hydrogels, as well as fast digestion rate, was identified. Biocompatibility of the material system was confirmed by embedding of human umbilical vein endothelial cells with followed enzymatic release. As a proof-of-concept, we bioprinted vascular models containing perfusable, endothelial cell-lined channels that remained stable for 28 days in culture. Our work establishes digestible sacrificial biomaterials as a new material strategy for 3D bioprinting of complex tissue models.
中文翻译:

通过基于多材料投影的立体光刻技术,使用可酶降解的生物墨水进行血管生物打印
将腔体和通道引入3D生物打印构造中是重建生理组织架构和整合脉管系统的前提。基于投影的立体光刻技术固有地提供了高打印速度和高空间分辨率,但是到目前为止,尚无法制造出具有细胞和生物材料多样性的复杂的天然组织结构。牺牲性光墨的使用,即可在印刷后去除的可光聚合的生物材料,理论上允许通过多材料印刷工艺创建任何构造几何形状。然而,由于难以实施的技术和缺乏高性能的生物材料,实现该策略一直具有挑战性。在这项工作中,我们使用基于投影的 多材料立体光刻生物打印机和可酶降解的牺牲性光电墨水,以克服当前的障碍。通过透明质酸酶筛选了多种透明质酸基光油墨的可印刷性,机械性能和可消化性。鉴定出满足所有主要要求的制剂,即所需的印刷性能,足够坚固的水凝胶的产生以及快速的消化速率。通过嵌入人脐静脉内皮细胞并随后进行酶促释放,证实了材料系统的生物相容性。作为概念验证,我们对包含可灌注的内皮细胞内衬通道的血管模型进行了生物打印,该通道在培养中保持稳定28天。











































 京公网安备 11010802027423号
京公网安备 11010802027423号