Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P-stereocontrolled synthesis of oligo(nucleoside N3′→O5′ phosphoramidothioate)s – opportunities and limitations
RSC Advances ( IF 3.9 ) Pub Date : 2020-9-23 , DOI: 10.1039/d0ra04987e Ewa Radzikowska 1 , Renata Kaczmarek 1 , Dariusz Korczyński 1 , Agnieszka Krakowiak 1 , Barbara Mikołajczyk 1 , Janina Baraniak 1 , Piotr Guga 1 , Kraig A Wheeler 2 , Tomasz Pawlak 1 , Barbara Nawrot 1
RSC Advances ( IF 3.9 ) Pub Date : 2020-9-23 , DOI: 10.1039/d0ra04987e Ewa Radzikowska 1 , Renata Kaczmarek 1 , Dariusz Korczyński 1 , Agnieszka Krakowiak 1 , Barbara Mikołajczyk 1 , Janina Baraniak 1 , Piotr Guga 1 , Kraig A Wheeler 2 , Tomasz Pawlak 1 , Barbara Nawrot 1
Affiliation
|
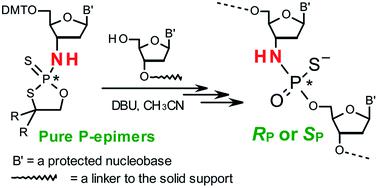
3′-N-(2-Thio-1,3,2-oxathiaphospholane) derivatives of 5′-O-DMT-3′-amino-2′,3′-dideoxy-ribonucleosides (NOTP-N), that bear a 4,4-unsubstituted, 4,4-dimethyl, or 4,4-pentamethylene substituted oxathiaphospholane ring, were synthesized. Within these three series, NOTP-N differed by canonical nucleobases (i.e., AdeBz, CytBz, GuaiBu, or Thy). The monomers were chromatographically separated into P-diastereomers, which were further used to prepare NNPSN′ dinucleotides (3), as well as short P-stereodefined oligo(deoxyribonucleoside N3′→O5′ phosphoramidothioate)s (NPS-) and chimeric NPS/PO- and NPS/PS-oligomers. The condensation reaction for NOTP-N monomers was found to be 5–6 times slower than the analogous OTP derivatives. When the 5′-end nucleoside of a growing oligomer adopts a C3′-endo conformation, a conformational ‘clash’ with the incoming NOTP-N monomer takes place, which is a main factor decreasing the repetitive yield of chain elongation. Although both isomers of NNPSN′ were digested by the HINT1 phosphoramidase enzyme, the isomers hydrolyzed at a faster rate were tentatively assigned the RP absolute configuration. This assignment is supported by X-ray analysis of the protected dinucleotide DMTdGiBuNPSMeTOAc, which is P-stereoequivalent to the hydrolyzed faster P-diastereomer of dGNPST.
中文翻译:

寡聚(核苷 N3′→O5′ 硫代磷酰胺)的 P 立体控制合成 – 机会和局限性
5'- O -DMT-3'-氨基-2',3'-二脱氧核糖核苷( N OTP-N) 的3'- N -(2-硫代-1,3,2-氧硫杂磷杂环戊烷) 衍生物,具有合成了4,4-未取代、4,4-二甲基或4,4-五亚甲基取代的氧硫杂磷杂环戊烷环。在这三个系列中,N OTP-N 因典型核碱基(即Ade Bz、 Cyt Bz、 Gua iBu或 Thy)而有所不同。单体经色谱分离为 P-非对映异构体,进一步用于制备 N NPS N' 二核苷酸 ( 3 ),以及短 P-立体寡聚(脱氧核糖核苷 N3'→O5' 硫代磷酰胺) (NPS-) 和嵌合 NPS /PO- 和 NPS/PS- 低聚物。N OTP-N 单体的缩合反应比类似的 OTP 衍生物慢 5-6 倍。当生长寡聚体的5'端核苷采用C3'-内构象时,与引入的N OTP-N单体发生构象“冲突” ,这是降低链延伸重复产量的主要因素。尽管 N NPS N' 的两种异构体均被 HINT1 磷酸酰胺酶消化,但以较快的速率水解的异构体暂时被指定为R P绝对构型。这一分配得到了受保护的二核苷酸DMT dG iBu NPSMe T OAc的 X 射线分析的支持,该二核苷酸与 dG NPS T的水解速度更快的 P-非对映异构体是 P-立体等效的。
更新日期:2020-09-23
中文翻译:

寡聚(核苷 N3′→O5′ 硫代磷酰胺)的 P 立体控制合成 – 机会和局限性
5'- O -DMT-3'-氨基-2',3'-二脱氧核糖核苷( N OTP-N) 的3'- N -(2-硫代-1,3,2-氧硫杂磷杂环戊烷) 衍生物,具有合成了4,4-未取代、4,4-二甲基或4,4-五亚甲基取代的氧硫杂磷杂环戊烷环。在这三个系列中,N OTP-N 因典型核碱基(即Ade Bz、 Cyt Bz、 Gua iBu或 Thy)而有所不同。单体经色谱分离为 P-非对映异构体,进一步用于制备 N NPS N' 二核苷酸 ( 3 ),以及短 P-立体寡聚(脱氧核糖核苷 N3'→O5' 硫代磷酰胺) (NPS-) 和嵌合 NPS /PO- 和 NPS/PS- 低聚物。N OTP-N 单体的缩合反应比类似的 OTP 衍生物慢 5-6 倍。当生长寡聚体的5'端核苷采用C3'-内构象时,与引入的N OTP-N单体发生构象“冲突” ,这是降低链延伸重复产量的主要因素。尽管 N NPS N' 的两种异构体均被 HINT1 磷酸酰胺酶消化,但以较快的速率水解的异构体暂时被指定为R P绝对构型。这一分配得到了受保护的二核苷酸DMT dG iBu NPSMe T OAc的 X 射线分析的支持,该二核苷酸与 dG NPS T的水解速度更快的 P-非对映异构体是 P-立体等效的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号