Current Protein & Peptide Science ( IF 1.9 ) Pub Date : 2020-06-30 , DOI: 10.2174/1389203721666200318161330 Sebastian Kwiatkowski 1 , Jakub Drozak 1
|
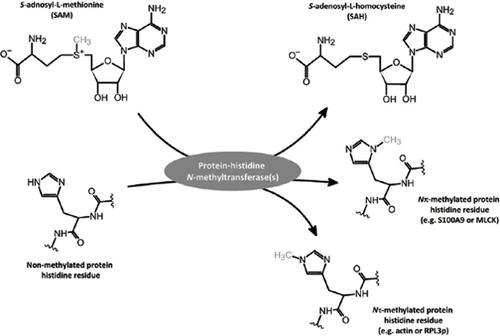
Protein histidine methylation is a rarely studied posttranslational modification in eukaryotes. Although the presence of N-methylhistidine was demonstrated in actin in the early 1960s, so far, only a limited number of proteins containing N-methylhistidine have been reported, including S100A9, myosin, skeletal muscle myosin light chain kinase (MLCK 2), and ribosomal protein Rpl3. Furthermore, the role of histidine methylation in the functioning of the protein and in cell physiology remains unclear due to a shortage of studies focusing on this topic. However, the molecular identification of the first two distinct histidine-specific protein methyltransferases has been established in yeast (Hpm1) and in metazoan species (actin-histidine N-methyltransferase), giving new insights into the phenomenon of protein methylation at histidine sites. As a result, we are now beginning to recognize protein histidine methylation as an important regulatory mechanism of protein functioning whose loss may have deleterious consequences in both cells and in organisms. In this review, we aim to summarize the recent advances in the understanding of the chemical, enzymological, and physiological aspects of protein histidine methylation.
中文翻译:

蛋白组氨酸甲基化。
组蛋白甲基化是真核生物中很少研究的翻译后修饰。尽管肌动蛋白在1960年代初期就证明了N-甲基组氨酸的存在,但到目前为止,仅报道了数量有限的含有N-甲基组氨酸的蛋白质,包括S100A9,肌球蛋白,骨骼肌肌球蛋白轻链激酶(MLCK 2)和核糖体蛋白Rpl3。此外,由于缺乏针对该主题的研究,组氨酸甲基化在蛋白质功能和细胞生理中的作用仍不清楚。但是,已经在酵母(Hpm1)和后生动物物种(肌动蛋白-组氨酸N-甲基转移酶)中建立了前两种不同的组氨酸特异性蛋白甲基转移酶的分子鉴定,从而为组氨酸位点蛋白质甲基化现象提供了新的见解。结果是,我们现在开始认识到蛋白质组氨酸甲基化是蛋白质功能的重要调节机制,蛋白质的丢失可能对细胞和生物体均造成有害后果。在这篇综述中,我们旨在总结蛋白质组氨酸甲基化的化学,酶学和生理学方面的最新进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号