当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Invoking Side-Chain Functionality for the Mediation of Regioselectivity during Ring-Opening Polymerization of Glucose Carbonates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-23 , DOI: 10.1021/jacs.0c05610 Yue Song , Xin Yang 1 , Yidan Shen , Mei Dong , Yen-Nan Lin 2 , Michael B Hall 1 , Karen L Wooley
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-23 , DOI: 10.1021/jacs.0c05610 Yue Song , Xin Yang 1 , Yidan Shen , Mei Dong , Yen-Nan Lin 2 , Michael B Hall 1 , Karen L Wooley
Affiliation
|
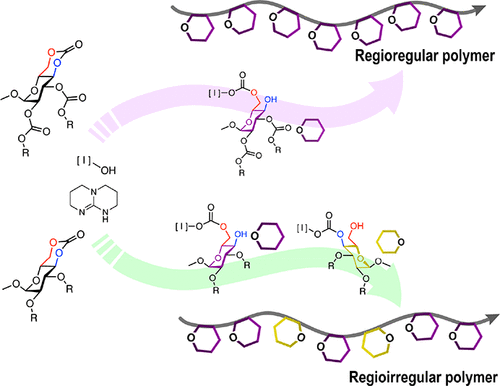
The extent of participation of side-chain functionalities during the 1,5,7-triazabicyclo[5.4.0]dec-5-ene (TBD) organobase-catalyzed ring-opening polymerizations (ROP) of six-membered cyclic d-glucose-based carbonates was found to result in significantly different regiochemical outcomes. High regioselectivity was observed for naturally derived poly(4,6-d-glucose carbonate)s (PGCs) containing carbonate side chain substituents in the 2- and 3-positions, whereas regioirregularity was found for analogous PGCs with ether side-chain substituents. The backbone connectivities and structural details of these PGCs were examined through a combination of comprehensive 1D and 2D NMR studies on unimers and dimers, verifying the ring-opening preferences and indicating the contribution of side-chain functionalities in regioselective ROP processes. A molecular understanding of the curious role of side-chain functionalities was demonstrated via density functional theory calculations, revealing stabilization effects of intermolecular hydrogen bonding between the side-chain functionalities and TBD in the transition states. Overall, this work provides fundamental insights into the organocatalytic ROP of these specific six-membered asymmetric cyclic glucose carbonates. More importantly, these findings serve as a foundation for future design strategies that incorporate adjacent functionalities within monomers to act as directing groups and impart molecular interactions that define regiochemical ring-opening.
中文翻译:

在葡萄糖碳酸酯的开环聚合过程中调用侧链功能来调节区域选择性
在 1,5,7-三氮杂双环 [5.4.0]dec-5-烯 (TBD) 有机碱催化的六元环状 d-葡萄糖开环聚合 (ROP) 中侧链官能团的参与程度-发现基碳酸盐导致显着不同的区域化学结果。对于在 2 位和 3 位含有碳酸酯侧链取代基的天然衍生聚(4,6-d-葡萄糖碳酸酯)(PGC),观察到高区域选择性,而在具有醚侧链取代基的类似 PGC 中发现了区域不规则性。这些 PGC 的骨架连接性和结构细节通过对单聚体和二聚体的综合 1D 和 2D NMR 研究的组合进行检查,验证开环偏好并表明侧链功能在区域选择性 ROP 过程中的贡献。通过密度泛函理论计算证明了对侧链官能团奇怪作用的分子理解,揭示了侧链官能团与过渡态 TBD 之间的分子间氢键的稳定作用。总的来说,这项工作提供了对这些特定六元不对称环状葡萄糖碳酸酯的有机催化 ROP 的基本见解。更重要的是,这些发现为未来的设计策略奠定了基础,这些策略将单体内的相邻功能结合起来作为导向基团,并赋予定义区域化学开环的分子相互作用。揭示了过渡态中侧链官能团和 TBD 之间的分子间氢键的稳定作用。总的来说,这项工作提供了对这些特定六元不对称环状葡萄糖碳酸酯的有机催化 ROP 的基本见解。更重要的是,这些发现为未来的设计策略奠定了基础,这些策略将单体内的相邻功能结合起来作为导向基团,并赋予定义区域化学开环的分子相互作用。揭示了过渡态中侧链官能团和 TBD 之间的分子间氢键的稳定作用。总的来说,这项工作提供了对这些特定六元不对称环状葡萄糖碳酸酯的有机催化 ROP 的基本见解。更重要的是,这些发现为未来的设计策略奠定了基础,这些策略将单体内的相邻功能结合起来作为导向基团,并赋予定义区域化学开环的分子相互作用。
更新日期:2020-09-23
中文翻译:

在葡萄糖碳酸酯的开环聚合过程中调用侧链功能来调节区域选择性
在 1,5,7-三氮杂双环 [5.4.0]dec-5-烯 (TBD) 有机碱催化的六元环状 d-葡萄糖开环聚合 (ROP) 中侧链官能团的参与程度-发现基碳酸盐导致显着不同的区域化学结果。对于在 2 位和 3 位含有碳酸酯侧链取代基的天然衍生聚(4,6-d-葡萄糖碳酸酯)(PGC),观察到高区域选择性,而在具有醚侧链取代基的类似 PGC 中发现了区域不规则性。这些 PGC 的骨架连接性和结构细节通过对单聚体和二聚体的综合 1D 和 2D NMR 研究的组合进行检查,验证开环偏好并表明侧链功能在区域选择性 ROP 过程中的贡献。通过密度泛函理论计算证明了对侧链官能团奇怪作用的分子理解,揭示了侧链官能团与过渡态 TBD 之间的分子间氢键的稳定作用。总的来说,这项工作提供了对这些特定六元不对称环状葡萄糖碳酸酯的有机催化 ROP 的基本见解。更重要的是,这些发现为未来的设计策略奠定了基础,这些策略将单体内的相邻功能结合起来作为导向基团,并赋予定义区域化学开环的分子相互作用。揭示了过渡态中侧链官能团和 TBD 之间的分子间氢键的稳定作用。总的来说,这项工作提供了对这些特定六元不对称环状葡萄糖碳酸酯的有机催化 ROP 的基本见解。更重要的是,这些发现为未来的设计策略奠定了基础,这些策略将单体内的相邻功能结合起来作为导向基团,并赋予定义区域化学开环的分子相互作用。揭示了过渡态中侧链官能团和 TBD 之间的分子间氢键的稳定作用。总的来说,这项工作提供了对这些特定六元不对称环状葡萄糖碳酸酯的有机催化 ROP 的基本见解。更重要的是,这些发现为未来的设计策略奠定了基础,这些策略将单体内的相邻功能结合起来作为导向基团,并赋予定义区域化学开环的分子相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号