当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination, Correlation, and Thermodynamic Analysis of the Solid–Liquid Phase Equilibrium of 1,4-Dicyanobenzene in Pure Solvents at Various Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.jced.0c00604 Yajun Li 1 , Chuanli Lu 1 , Ruirong Chen 1 , Kui Wu 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.jced.0c00604 Yajun Li 1 , Chuanli Lu 1 , Ruirong Chen 1 , Kui Wu 2
Affiliation
|
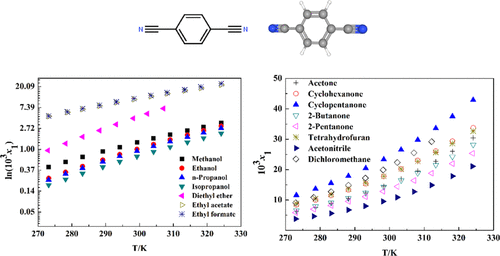
The solubility of 1,4-dicyanobenzene (DCB) in 17 neat solvents, that is, methanol, ethanol, n-propanol, isopropanol, acetone, ethyl acetate, ethyl formate, methyl acetate, cyclohexanone, cyclopentanone, tetrahydrofuran, 2-butanone, acetonitrile, chloroform, 2-pentanone, dichloromethane, and diethyl ether, was measured via a gravimetric method from 272.95 to 324.15 K under ambient pressure. Within the experimental temperature range, the mole fraction solubility of DCB increased with the rising temperature in all selected solvents, and the order of magnitude is cyclopentanone > dichloromethane > cyclohexanone ≈ tetrahydrofuran > chloroform > (acetone, 2-butanone) > 2-pentanone ≈ methyl acetate > ethyl formate > ethyl acetate > acetonitrile > diethyl ether > methanol > ethanol > n-propanol > isopropanol. The solubility of DCB in ketones was much higher than that in ethers and alcohols. The Wilson model, nonrandom two-liquid model, Apelblat equation, and λh equation were employed to mathematically express the solid–liquid phase equilibrium data of DCB. The maximum values of the root-mean-square and relative average deviations were 9.83 × 10–5 and 5.21 × 10–3, respectively, which indicated that good correlation was recorded between the experimental and calculated data. The dissolution enthalpy, entropy, and Gibbs free energy were calculated on the basis of thermodynamic relations and the Wilson model, which revealed that the dissolution of DCB was a spontaneous and endothermic process.
中文翻译:

不同温度下纯溶剂中1,4-二氰基苯的固液相平衡的测定,相关和热力学分析
1,4-二氰基苯(DCB)在17种纯溶剂中的溶解度,即甲醇,乙醇,正丙醇,异丙醇,丙酮,乙酸乙酯,甲酸乙酯,乙酸甲酯,环己酮,环戊酮,四氢呋喃,2-丁酮,乙腈,氯仿,2-戊酮,二氯甲烷和乙醚在环境压力下通过重量法从272.95至324.15 K测量。在实验温度范围内,在所有选定的溶剂中,DCB的摩尔分数溶解度均随温度的升高而增加,其数量级为环戊酮>二氯甲烷>环己酮≈四氢呋喃>氯仿>(丙酮,2-丁酮)> 2-戊酮≈乙酸甲酯>甲酸乙酯>乙酸乙酯>乙腈>乙醚>甲醇>乙醇>-丙醇>异丙醇。DCB在酮中的溶解度比在醚和醇中的溶解度高得多。威尔逊模型,非随机双液体模型,Apelblat方程,和λ ħ方程被用来在数学上表达DCB的固-液相平衡数据。均方根和相对平均偏差的最大值分别为9.83×10 –5和5.21×10 –3,这表明在实验数据和计算数据之间记录了良好的相关性。根据热力学关系和威尔逊模型计算了溶解焓,熵和吉布斯自由能,表明DCB的溶解是一个自发的吸热过程。
更新日期:2020-10-08
中文翻译:

不同温度下纯溶剂中1,4-二氰基苯的固液相平衡的测定,相关和热力学分析
1,4-二氰基苯(DCB)在17种纯溶剂中的溶解度,即甲醇,乙醇,正丙醇,异丙醇,丙酮,乙酸乙酯,甲酸乙酯,乙酸甲酯,环己酮,环戊酮,四氢呋喃,2-丁酮,乙腈,氯仿,2-戊酮,二氯甲烷和乙醚在环境压力下通过重量法从272.95至324.15 K测量。在实验温度范围内,在所有选定的溶剂中,DCB的摩尔分数溶解度均随温度的升高而增加,其数量级为环戊酮>二氯甲烷>环己酮≈四氢呋喃>氯仿>(丙酮,2-丁酮)> 2-戊酮≈乙酸甲酯>甲酸乙酯>乙酸乙酯>乙腈>乙醚>甲醇>乙醇>-丙醇>异丙醇。DCB在酮中的溶解度比在醚和醇中的溶解度高得多。威尔逊模型,非随机双液体模型,Apelblat方程,和λ ħ方程被用来在数学上表达DCB的固-液相平衡数据。均方根和相对平均偏差的最大值分别为9.83×10 –5和5.21×10 –3,这表明在实验数据和计算数据之间记录了良好的相关性。根据热力学关系和威尔逊模型计算了溶解焓,熵和吉布斯自由能,表明DCB的溶解是一个自发的吸热过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号