当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent Cage Effects: A Comparison of Geminate and Nongeminate Radical Cage Pair Combination Efficiencies.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.inorgchem.0c01441 Justin T Barry 1 , David R Tyler 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.inorgchem.0c01441 Justin T Barry 1 , David R Tyler 1
Affiliation
|
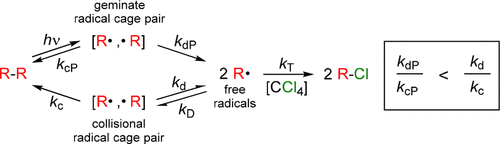
This study measured and compared the combination efficiencies (FcP) of geminate radical cage pairs to nongeminate (collisional) radical cage pairs (Fc′). For the [Cp′(CO)3Mo·, ·Mo(CO)3Cp′] radical cage pair, Fc′ was found to be smaller than FcP in solutions having the same viscosity. It is proposed that the difference in FcP and Fc′ arises because the radicals in the collisional cage pair are less likely to have the correct orbital orientation for radical–radical combination to occur, whereas photochemically generated geminate cage pairs are more likely to have the correct orbital orientation. As predicted, FcP and Fc′ both increase when the microviscosity of a solution increases.
中文翻译:

溶剂笼效应:自由基和非自由基自由基笼对组合效率的比较。
本研究中测量并比较该组合效率(˚F厘泊成双自由基笼对)至nongeminate(碰撞)自由基笼双(˚F Ç ')。对于[Cp'(CO)3 Mo·,·Mo(CO)3 Cp']自由基笼对,在具有相同粘度的溶液中,发现F c '小于F cP。提议F cP和F c的差异之所以出现“,是因为碰撞笼对中的自由基不太可能具有发生自由基-自由基组合的正确轨道方向,而光化学生成的双联笼对则更可能具有正确的轨道方向。如所预测的,当溶液的微粘度增加时,F cP和F c '都增加。
更新日期:2020-10-05
中文翻译:

溶剂笼效应:自由基和非自由基自由基笼对组合效率的比较。
本研究中测量并比较该组合效率(˚F厘泊成双自由基笼对)至nongeminate(碰撞)自由基笼双(˚F Ç ')。对于[Cp'(CO)3 Mo·,·Mo(CO)3 Cp']自由基笼对,在具有相同粘度的溶液中,发现F c '小于F cP。提议F cP和F c的差异之所以出现“,是因为碰撞笼对中的自由基不太可能具有发生自由基-自由基组合的正确轨道方向,而光化学生成的双联笼对则更可能具有正确的轨道方向。如所预测的,当溶液的微粘度增加时,F cP和F c '都增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号