当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vivo Targeting Using Arylboronate / Nopoldiol Click Conjugation.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.bioconjchem.0c00453 Sandeep Palvai 1 , Jasmine Bhangu 2 , Burcin Akgun 2 , Christopher T Moody 1 , Dennis G Hall 2 , Yevgeny Brudno 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.bioconjchem.0c00453 Sandeep Palvai 1 , Jasmine Bhangu 2 , Burcin Akgun 2 , Christopher T Moody 1 , Dennis G Hall 2 , Yevgeny Brudno 1
Affiliation
|
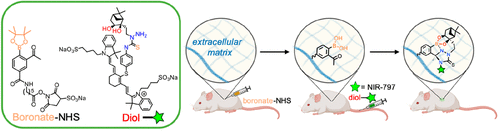
Bioorthogonal click reactions yielding stable and irreversible adducts are in high demand for in vivo applications, including in biomolecular labeling, diagnostic imaging, and drug delivery. Previously, we reported a novel bioorthogonal “click” reaction based on the coupling of ortho-acetyl arylboronates and thiosemicarbazide-functionalized nopoldiol. We now report that a detailed structural analysis of the arylboronate/nopoldiol adduct by X-ray crystallography and 11B NMR reveals that the bioorthogonal reactants form, unexpectedly, a tetracyclic adduct through the cyclization of the distal nitrogen into the semithiocarbazone leading to a strong B–N dative bond and two new 5-membered rings. The cyclization adduct, which protects the boronate unit against hydrolytic breakdown, sheds light on the irreversible nature of this polycondensation. The potential of this reaction to work in a live animal setting was studied through in vivo capture of fluorescently labeled molecules in vivo. Arylboronates were introduced into tissues through intradermal injection of their activated NHS esters, which react with amines in the extracellular matrix. Fluorescently labeled nopoldiol molecules were administered systemically and were efficiently captured by the arylboronic acids in a location-specific manner. Taken together, these in vivo proof-of-concept studies establish arylboronate/nopoldiol bioorthogonal chemistry as a candidate for wide array of applications in chemical biology and drug delivery.
中文翻译:

使用芳基硼酸酯 / Nopoldiol Click 偶联进行体内靶向。
产生稳定和不可逆加合物的生物正交点击反应在体内应用中有很高的需求,包括生物分子标记、诊断成像和药物递送。之前,我们报道了一种基于邻乙酰基芳基硼酸酯和氨基硫脲功能化的酚醛二醇偶联的新型生物正交“点击”反应。我们现在报告了通过 X 射线晶体学和11B NMR 显示,生物正交反应物意外地通过远端氮环化成半硫脲形成四环加合物,导致强 B-N 配价键和两个新的 5 元环。环化加合物保护硼酸酯单元免受水解分解,揭示了这种缩聚的不可逆性质。通过在体内捕获荧光标记分子,研究了该反应在活体动物环境中发挥作用的潜力. 芳基硼酸酯通过皮内注射活化的 NHS 酯被引入组织,这些酯与细胞外基质中的胺反应。荧光标记的 nopoldiol 分子被全身给药,并被芳基硼酸以特定于位置的方式有效捕获。综上所述,这些体内概念验证研究确立了芳基硼酸酯/壬二醇生物正交化学作为化学生物学和药物递送中广泛应用的候选者。
更新日期:2020-10-21
中文翻译:

使用芳基硼酸酯 / Nopoldiol Click 偶联进行体内靶向。
产生稳定和不可逆加合物的生物正交点击反应在体内应用中有很高的需求,包括生物分子标记、诊断成像和药物递送。之前,我们报道了一种基于邻乙酰基芳基硼酸酯和氨基硫脲功能化的酚醛二醇偶联的新型生物正交“点击”反应。我们现在报告了通过 X 射线晶体学和11B NMR 显示,生物正交反应物意外地通过远端氮环化成半硫脲形成四环加合物,导致强 B-N 配价键和两个新的 5 元环。环化加合物保护硼酸酯单元免受水解分解,揭示了这种缩聚的不可逆性质。通过在体内捕获荧光标记分子,研究了该反应在活体动物环境中发挥作用的潜力. 芳基硼酸酯通过皮内注射活化的 NHS 酯被引入组织,这些酯与细胞外基质中的胺反应。荧光标记的 nopoldiol 分子被全身给药,并被芳基硼酸以特定于位置的方式有效捕获。综上所述,这些体内概念验证研究确立了芳基硼酸酯/壬二醇生物正交化学作为化学生物学和药物递送中广泛应用的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号