Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mammalian Expression and In Situ Biotinylation of Extracellular Protein Targets for Directed Evolution
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-22 , DOI: 10.1021/acsomega.0c03990 Brian J. Grindel 1 , Brian J. Engel 1 , Carolyn G. Hall 2 , Lindsay E. Kelderhouse 1 , Anthony Lucci 2 , Niki M. Zacharias 3 , Terry T. Takahashi 4 , Steven W. Millward 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-22 , DOI: 10.1021/acsomega.0c03990 Brian J. Grindel 1 , Brian J. Engel 1 , Carolyn G. Hall 2 , Lindsay E. Kelderhouse 1 , Anthony Lucci 2 , Niki M. Zacharias 3 , Terry T. Takahashi 4 , Steven W. Millward 1
Affiliation
|
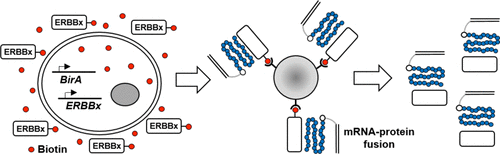
Directed evolution is a powerful tool for the selection of functional ligands from molecular libraries. Extracellular domains (ECDs) of cell surface receptors are common selection targets for therapeutic and imaging agent development. Unfortunately, these proteins are often post-translationally modified and are therefore unsuitable for expression in bacterial systems. Directional immobilization of these targets is further hampered by the absence of biorthogonal groups for site-specific chemical conjugation. We have developed a nonadherent mammalian expression system for rapid, high-yield expression of biotinylated ECDs. ECDs from EGFR, HER2, and HER3 were site-specifically biotinylated in situ and recovered from the cell culture supernatant with yields of up to 10 mg/L at >90% purity. Biotinylated ECDs also contained a protease cleavage site for rapid and selective release of the ECD after immobilization on avidin/streptavidin resins and library binding. A model mRNA display selection round was carried out against the HER2 ECD with the HER2 affibody expressed as an mRNA–protein fusion. HER2 affibody–mRNA fusions were selectively released by thrombin and quantitative PCR revealed substantial improvements in the enrichment of functional affibody–mRNA fusions relative to direct PCR amplification of the resin-bound target. This methodology allows rapid purification of high-quality targets for directed evolution and selective elution of functional sequences at the conclusion of each selection round.
中文翻译:

定向表达的细胞外蛋白靶标的哺乳动物表达和原位生物素化。
定向进化是从分子库中选择功能性配体的有力工具。细胞表面受体的细胞外结构域(ECD)是治疗和成像剂开发的常见选择目标。不幸的是,这些蛋白质经常被翻译后修饰,因此不适合在细菌系统中表达。这些靶标的定向固定进一步由于缺少用于位点特异性化学缀合的双正交基团而进一步受到阻碍。我们已经开发了一种非粘附性哺乳动物表达系统,用于生物素化ECD的快速,高产量表达。来自EGFR,HER2和HER3的ECD经过原位特异性生物素化并从细胞培养上清液中回收,纯度> 90%时产率高达10 mg / L。生物素化的ECD还包含一个蛋白酶切割位点,用于固定在亲和素/链霉亲和素树脂上并与文库结合后,ECD可以快速选择性地释放。针对HER2 ECD进行了模型mRNA显示选择回合,HER2亲和体表达为mRNA-蛋白融合体。HER2亲和体-mRNA融合物被凝血酶选择性释放,定量PCR显示,相对于直接结合树脂结合靶标的PCR扩增,功能性亲和体-mRNA融合体的富集度显着提高。这种方法可以在每个选择周期结束时快速纯化高质量的靶标,用于定向进化和功能序列的选择性洗脱。
更新日期:2020-10-06
中文翻译:

定向表达的细胞外蛋白靶标的哺乳动物表达和原位生物素化。
定向进化是从分子库中选择功能性配体的有力工具。细胞表面受体的细胞外结构域(ECD)是治疗和成像剂开发的常见选择目标。不幸的是,这些蛋白质经常被翻译后修饰,因此不适合在细菌系统中表达。这些靶标的定向固定进一步由于缺少用于位点特异性化学缀合的双正交基团而进一步受到阻碍。我们已经开发了一种非粘附性哺乳动物表达系统,用于生物素化ECD的快速,高产量表达。来自EGFR,HER2和HER3的ECD经过原位特异性生物素化并从细胞培养上清液中回收,纯度> 90%时产率高达10 mg / L。生物素化的ECD还包含一个蛋白酶切割位点,用于固定在亲和素/链霉亲和素树脂上并与文库结合后,ECD可以快速选择性地释放。针对HER2 ECD进行了模型mRNA显示选择回合,HER2亲和体表达为mRNA-蛋白融合体。HER2亲和体-mRNA融合物被凝血酶选择性释放,定量PCR显示,相对于直接结合树脂结合靶标的PCR扩增,功能性亲和体-mRNA融合体的富集度显着提高。这种方法可以在每个选择周期结束时快速纯化高质量的靶标,用于定向进化和功能序列的选择性洗脱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号