当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent Advances in Catalytic Enantioselective Synthesis of Fluorinated α‐ and β‐Amino Acids
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-22 , DOI: 10.1002/adsc.202000966 Xue-Xin Zhang 1 , Yang Gao 1 , Xiao-Si Hu 1 , Cong-Bin Ji 2 , Yun-Lin Liu 3 , Jin-Sheng Yu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-22 , DOI: 10.1002/adsc.202000966 Xue-Xin Zhang 1 , Yang Gao 1 , Xiao-Si Hu 1 , Cong-Bin Ji 2 , Yun-Lin Liu 3 , Jin-Sheng Yu 1
Affiliation
|
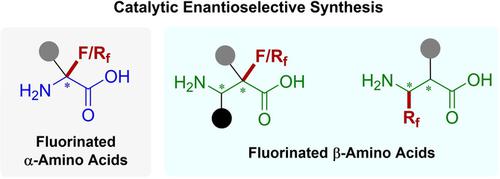
Enantioenriched fluorinated α‐ and β‐amino acids are often encountered in numerous pharmaceuticals and bioactive molecules, and also of great importance as probes in PET and NMR for studying the behavior of enzymes and for incorporation into peptides and drug candidates. Among various synthetic strategies developed, catalytic enantioselective synthesis proves to be one of the most facile and powerful protocols to construct such privileged structures. The past decade has witnessed considerable progress in the catalytic enantioselective construction of chiral fluorinated α‐ and β‐amino acid derivatives with structural diversity. In this review, we summarize these impressive achievements according to the bond‐forming way of fluorinated α‐ or β‐amino acids, respectively, and underline the remaining challenges. This information would provide important guidance and some inspiration for the researchers engaged in organic fluorine and medicinal chemistry.
中文翻译:

氟化α-和β-氨基酸催化对映选择性合成的最新进展
在许多药物和生物活性分子中经常会遇到对映体富集的氟化α-和β-氨基酸,作为PET和NMR中的探针,对于研究酶的行为以及掺入肽和候选药物中也非常重要。在开发的各种合成策略中,催化对映选择性合成被证明是构建这种特权结构的最简便,最有效的方法之一。在过去的十年中,具有结构多样性的手性氟化α-和β-氨基酸衍生物的催化对映选择性构建取得了长足进展。在这篇综述中,我们分别根据氟化α-或β-氨基酸的键形成方式总结了这些令人印象深刻的成就,并着重指出了仍然存在的挑战。
更新日期:2020-11-19
中文翻译:

氟化α-和β-氨基酸催化对映选择性合成的最新进展
在许多药物和生物活性分子中经常会遇到对映体富集的氟化α-和β-氨基酸,作为PET和NMR中的探针,对于研究酶的行为以及掺入肽和候选药物中也非常重要。在开发的各种合成策略中,催化对映选择性合成被证明是构建这种特权结构的最简便,最有效的方法之一。在过去的十年中,具有结构多样性的手性氟化α-和β-氨基酸衍生物的催化对映选择性构建取得了长足进展。在这篇综述中,我们分别根据氟化α-或β-氨基酸的键形成方式总结了这些令人印象深刻的成就,并着重指出了仍然存在的挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号