当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of Class IIa HDACs improves endothelial barrier function in endotoxin‐induced acute lung injury
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-09-22 , DOI: 10.1002/jcp.30053 Anita Kovacs-Kasa 1 , Laszlo Kovacs 2 , Mary Cherian-Shaw 1 , Vijay Patel 3 , Mary L Meadows 1 , David J Fulton 1, 2 , Yunchao Su 1, 2, 4, 5 , Alexander D Verin 1, 4
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-09-22 , DOI: 10.1002/jcp.30053 Anita Kovacs-Kasa 1 , Laszlo Kovacs 2 , Mary Cherian-Shaw 1 , Vijay Patel 3 , Mary L Meadows 1 , David J Fulton 1, 2 , Yunchao Su 1, 2, 4, 5 , Alexander D Verin 1, 4
Affiliation
|
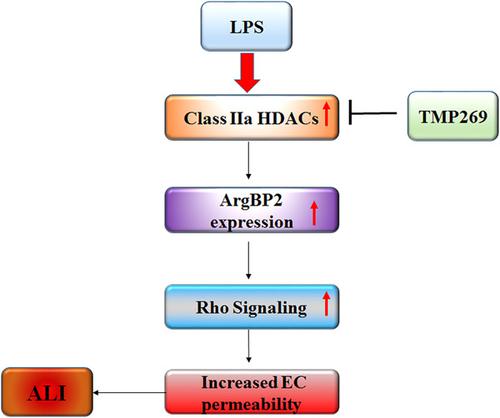
Acute lung injury (ALI) is an acute inflammatory process arises from a wide range of lung insults. A major cause of ALI is dysfunction of the pulmonary vascular endothelial barrier but the mechanisms involved are incompletely understood. The therapeutic potential of histone deacetylase (HDAC) inhibitors for the treatment of cardiovascular and inflammatory diseases is increasingly apparent, but the mechanisms by which HDACs regulate pulmonary vascular barrier function remain to be resolved. We found that specific Class IIa HDACs inhibitor, TMP269, significantly attenuated the lipopolysaccharide (LPS)‐induced human lung microvascular endothelial cells (HLMVEC) barrier compromise in vitro and improved vascular barrier integrity and lung function in murine model of ALI in vivo. TMP269 decreased LPS‐induced myosin light chain phosphorylation suggesting the role for Class IIa HDACs in LPS‐induced cytoskeleton reorganization. TMP269 did not affect microtubule structure and tubulin acetylation in contrast to the HDAC6‐specific inhibitor, Tubastatin A suggesting that Class IIa HDACs and HDAC6 (Class IIb) regulate endothelial cytoskeleton and permeability via different mechanisms. Furthermore, LPS increased the expression of ArgBP2 which has recently been attributed to HDAC‐mediated activation of Rho. Depletion of ArgBP2 abolished the ability of LPS to disrupt barrier function in HLMVEC and both TMP269 and Tubastatin A decreased the level of ArgBP2 expression after LPS stimulation suggesting that both Class IIa and IIb HDACs regulate endothelial permeability via ArgBP2‐dependent mechanism. Collectively, our data strongly suggest that Class IIa HDACs are involved in LPS‐induced ALI in vitro and in vivo via specific mechanism which involved contractile responses, but not microtubule reorganization.
中文翻译:

抑制 IIa 类 HDAC 可改善内毒素诱导的急性肺损伤中的内皮屏障功能
急性肺损伤(ALI)是由多种肺部损伤引起的急性炎症过程。 ALI 的一个主要原因是肺血管内皮屏障功能障碍,但其机制尚不完全清楚。组蛋白脱乙酰酶 (HDAC) 抑制剂治疗心血管和炎症性疾病的治疗潜力日益明显,但 HDAC 调节肺血管屏障功能的机制仍有待解决。我们发现,特定的 IIa 类 HDAC 抑制剂 TMP269 在体外显着减弱脂多糖 (LPS) 诱导的人肺微血管内皮细胞 (HLMVEC) 屏障受损,并在体内 ALI 小鼠模型中改善血管屏障完整性和肺功能。 TMP269 降低 LPS 诱导的肌球蛋白轻链磷酸化,表明 IIa 类 HDAC 在 LPS 诱导的细胞骨架重组中的作用。与 HDAC6 特异性抑制剂图巴他汀 A 相比,TMP269 不影响微管结构和微管蛋白乙酰化,这表明 IIa 类 HDAC 和 HDAC6(IIb 类)通过不同的机制调节内皮细胞骨架和通透性。此外,LPS 增加了 ArgBP2 的表达,最近将其归因于 HDAC 介导的 Rho 激活。 ArgBP2 的耗尽消除了 LPS 破坏 HLMVEC 屏障功能的能力,并且 TMP269 和图巴他汀 A 均在 LPS 刺激后降低了 ArgBP2 的表达水平,表明 IIa 类和 IIb 类 HDAC 均通过 ArgBP2 依赖性机制调节内皮通透性。 总的来说,我们的数据强烈表明,IIa 类 HDAC 通过涉及收缩反应但不涉及微管重组的特定机制参与体外和体内 LPS 诱导的 ALI。
更新日期:2020-09-22
中文翻译:

抑制 IIa 类 HDAC 可改善内毒素诱导的急性肺损伤中的内皮屏障功能
急性肺损伤(ALI)是由多种肺部损伤引起的急性炎症过程。 ALI 的一个主要原因是肺血管内皮屏障功能障碍,但其机制尚不完全清楚。组蛋白脱乙酰酶 (HDAC) 抑制剂治疗心血管和炎症性疾病的治疗潜力日益明显,但 HDAC 调节肺血管屏障功能的机制仍有待解决。我们发现,特定的 IIa 类 HDAC 抑制剂 TMP269 在体外显着减弱脂多糖 (LPS) 诱导的人肺微血管内皮细胞 (HLMVEC) 屏障受损,并在体内 ALI 小鼠模型中改善血管屏障完整性和肺功能。 TMP269 降低 LPS 诱导的肌球蛋白轻链磷酸化,表明 IIa 类 HDAC 在 LPS 诱导的细胞骨架重组中的作用。与 HDAC6 特异性抑制剂图巴他汀 A 相比,TMP269 不影响微管结构和微管蛋白乙酰化,这表明 IIa 类 HDAC 和 HDAC6(IIb 类)通过不同的机制调节内皮细胞骨架和通透性。此外,LPS 增加了 ArgBP2 的表达,最近将其归因于 HDAC 介导的 Rho 激活。 ArgBP2 的耗尽消除了 LPS 破坏 HLMVEC 屏障功能的能力,并且 TMP269 和图巴他汀 A 均在 LPS 刺激后降低了 ArgBP2 的表达水平,表明 IIa 类和 IIb 类 HDAC 均通过 ArgBP2 依赖性机制调节内皮通透性。 总的来说,我们的数据强烈表明,IIa 类 HDAC 通过涉及收缩反应但不涉及微管重组的特定机制参与体外和体内 LPS 诱导的 ALI。











































 京公网安备 11010802027423号
京公网安备 11010802027423号