当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
What determines the selectivity of arginine dihydroxylation by the nonheme iron enzyme OrfP?
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202004019 Hafiz Saqib Ali 1, 2 , Richard H Henchman 1, 2 , Sam P de Visser 1, 3
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-09-23 , DOI: 10.1002/chem.202004019 Hafiz Saqib Ali 1, 2 , Richard H Henchman 1, 2 , Sam P de Visser 1, 3
Affiliation
|
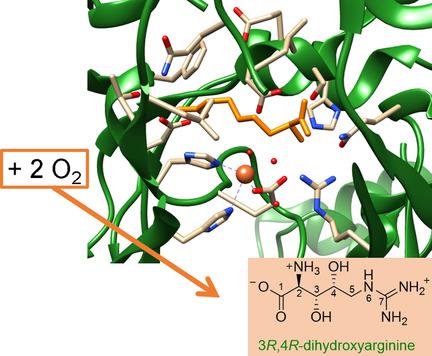
The nonheme iron enzyme OrfP reacts with l‐Arg selectively to form the 3R,4R‐dihydroxyarginine product, which in mammals can inhibit the nitric oxide synthase enzymes involved in blood pressure control. To understand the mechanisms of dioxygen activation of l‐Arg by OrfP and how it enables two sequential oxidation cycles on the same substrate, we performed a density functional theory study on a large active site cluster model. We show that substrate binding and positioning in the active site guides a highly selective reaction through C3−H hydrogen atom abstraction. This happens despite the fact that the C3−H and C4−H bond strengths of l‐Arg are very similar. Electronic differences in the two hydrogen atom abstraction pathways drive the reaction with an initial C3−H activation to a low‐energy 5σ‐pathway, while substrate positioning destabilizes the C4−H abstraction and sends it over the higher‐lying 5π‐pathway. We show that substrate and monohydroxylated products are strongly bound in the substrate binding pocket and hence product release is difficult and consequently its lifetime will be long enough to trigger a second oxygenation cycle.
中文翻译:

是什么决定了非血红素铁酶OrfP对精氨酸二羟基化反应的选择性?
非血红素铁酶OrfP与l -Arg选择性反应形成3 R,4 R-二羟基精氨酸产物,在哺乳动物中可抑制涉及血压控制的一氧化氮合酶。为了了解OrfP对l- Arg进行双氧激活的机制,以及它如何在同一底物上实现两个连续的氧化循环,我们对大型活性位点簇模型进行了密度泛函理论研究。我们表明,底物的结合和在活性位点上的定位通过C 3 -H氢原子抽象引导了高度选择性的反应。尽管存在以下事实,但仍会发生这种情况:C 3 -H和C 4 -H键强度为l‐Arg非常相似。在两个氢原子夺取电子通路差异驱使反应与初期的C 3 -H激活到低能量5 σ途径,而基板定位不稳定的C 4 -H抽象和在更高躺着将其发送5 π途径。我们显示底物和单羟基化产物牢固地结合在底物结合袋中,因此产物释放困难,因此其寿命将足够长以触发第二次氧合循环。
更新日期:2020-09-23
中文翻译:

是什么决定了非血红素铁酶OrfP对精氨酸二羟基化反应的选择性?
非血红素铁酶OrfP与l -Arg选择性反应形成3 R,4 R-二羟基精氨酸产物,在哺乳动物中可抑制涉及血压控制的一氧化氮合酶。为了了解OrfP对l- Arg进行双氧激活的机制,以及它如何在同一底物上实现两个连续的氧化循环,我们对大型活性位点簇模型进行了密度泛函理论研究。我们表明,底物的结合和在活性位点上的定位通过C 3 -H氢原子抽象引导了高度选择性的反应。尽管存在以下事实,但仍会发生这种情况:C 3 -H和C 4 -H键强度为l‐Arg非常相似。在两个氢原子夺取电子通路差异驱使反应与初期的C 3 -H激活到低能量5 σ途径,而基板定位不稳定的C 4 -H抽象和在更高躺着将其发送5 π途径。我们显示底物和单羟基化产物牢固地结合在底物结合袋中,因此产物释放困难,因此其寿命将足够长以触发第二次氧合循环。









































 京公网安备 11010802027423号
京公网安备 11010802027423号