当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α‐Methylene‐β‐Lactone Scaffold for Developing Chemical Probes at the Two Ends of the Selectivity Spectrum
ChemBioChem ( IF 2.6 ) Pub Date : 2020-09-22 , DOI: 10.1002/cbic.202000605 Lei Wang 1 , Louis P Riel 1 , Bekim Bajrami 2 , Bin Deng 3, 4 , Amy R Howell 1 , Xudong Yao 1, 5
ChemBioChem ( IF 2.6 ) Pub Date : 2020-09-22 , DOI: 10.1002/cbic.202000605 Lei Wang 1 , Louis P Riel 1 , Bekim Bajrami 2 , Bin Deng 3, 4 , Amy R Howell 1 , Xudong Yao 1, 5
Affiliation
|
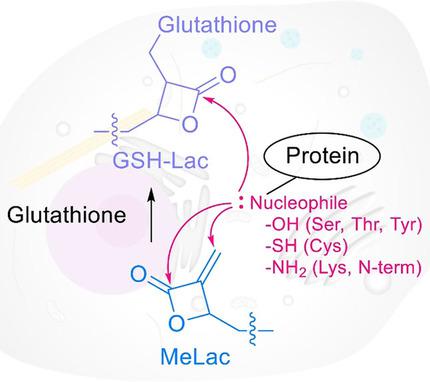
Create high‐coverage and selective probes from multi‐reactive scaffold: An α‐methylene‐β‐lactone (MeLac) scaffold provides new paths to create reactivity‐ and proximity‐driven covalent probes for chemical proteomics. Multiple reactivities of the MeLac warhead allows it to react with different protein nucleophiles through distinct mechanisms, and to assemble highly selective proteomics probes. MeLac‐derived probes have potential to make significant impacts on drug discovery and development.

中文翻译:

用于开发选择性谱两端化学探针的 α-亚甲基-β-内酯支架
从多反应支架创建高覆盖度和选择性探针:α-亚甲基-β-内酯 (MeLac) 支架为创建用于化学蛋白质组学的反应性和邻近驱动的共价探针提供了新途径。MeLac 弹头的多种反应性使其能够通过不同的机制与不同的蛋白质亲核试剂发生反应,并组装高选择性的蛋白质组探针。MeLac 衍生的探针有可能对药物发现和开发产生重大影响。

更新日期:2020-09-22

中文翻译:

用于开发选择性谱两端化学探针的 α-亚甲基-β-内酯支架
从多反应支架创建高覆盖度和选择性探针:α-亚甲基-β-内酯 (MeLac) 支架为创建用于化学蛋白质组学的反应性和邻近驱动的共价探针提供了新途径。MeLac 弹头的多种反应性使其能够通过不同的机制与不同的蛋白质亲核试剂发生反应,并组装高选择性的蛋白质组探针。MeLac 衍生的探针有可能对药物发现和开发产生重大影响。












































 京公网安备 11010802027423号
京公网安备 11010802027423号