当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The elucidation of phosphosugar stress response in Bacillus subtilis guides strain engineering for high N-acetylglucosamine production.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-09-23 , DOI: 10.1002/bit.27577 Tengfei Niu 1 , Xueqin Lv 1 , Yanfeng Liu 1 , Jianghua Li 2 , Guocheng Du 1 , Rodrigo Ledesma-Amaro 3 , Long Liu 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-09-23 , DOI: 10.1002/bit.27577 Tengfei Niu 1 , Xueqin Lv 1 , Yanfeng Liu 1 , Jianghua Li 2 , Guocheng Du 1 , Rodrigo Ledesma-Amaro 3 , Long Liu 1
Affiliation
|
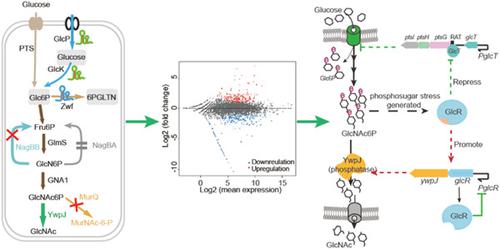
Bacillus subtilis is a preferred microbial host for the industrial production of nutraceuticals and a promising candidate for the synthesis of functional sugars, such as N‐acetylglucosamine (GlcNAc). Previously, a GlcNAc‐overproducer B. subtilis SFMI was constructed using glmS ribozyme dual‐regulatory tool. Herein, we further engineered to enhance carbon flux from glucose towards GlcNAc synthesis. As a result, the increased flux towards GlcNAc synthesis triggered phosphosugar stress response, which caused abnormal cell growth. Unfortunately, the mechanism of phosphosugar stress response had not been elucidated in B. subtilis. To reveal the stress mechanism and overcome its negative effect in bioproduction, we performed comparative transcriptome analysis. The results indicate that cells slow glucose utilization by repression of glucose import and accelerate catabolic reactions of phosphosugar. To verify these results, we overexpressed the phosphatase YwpJ, which relieved phosphosugar stress and allowed us to identify the enzyme responsible for GlcNAc synthesis from GlcNAc 6‐phosphate. In addition, the deletion of nagBB and murQ, responsible for GlcNAc precursor degradation, further improved GlcNAc synthesis. The best engineered strain, B. subtilis FMIP34, increased GlcNAc titer from 11.5 to 26.1 g/L in shake flasks and produced 87.5 g/L GlcNAc in 30‐L fed‐batch bioreactor. Our results not only elucidate, for the first time, the phosphosugar stress response mechanism in B. subtilis, but also demonstrate how the combination of rational metabolic engineering with novel insights into physiology and metabolism allows the construction of highly efficient microbial cell factories for the production of high‐value chemicals.
中文翻译:

枯草芽孢杆菌中磷糖应激反应的阐明指导了高 N-乙酰氨基葡萄糖生产的菌株工程。
枯草芽孢杆菌是营养食品工业生产的首选微生物宿主,也是合成功能性糖类(如N-乙酰氨基葡萄糖(GlcNAc))的有希望的候选者。以前,使用glmS核酶双调节工具构建了 GlcNAc 过量生产者B. subtilis SFMI 。在此,我们进一步设计以增强从葡萄糖到 GlcNAc 合成的碳通量。结果,增加的 GlcNAc 合成通量引发了磷糖应激反应,从而导致细胞生长异常。不幸的是,尚未阐明枯草芽孢杆菌中磷糖应激反应的机制. 为了揭示压力机制并克服其在生物生产中的负面影响,我们进行了比较转录组分析。结果表明细胞通过抑制葡萄糖输入来减缓葡萄糖利用并加速磷糖的分解代谢反应。为了验证这些结果,我们过度表达了磷酸酶 YwpJ,它减轻了磷酸糖压力,并允许我们鉴定负责从 GlcNAc 6-磷酸合成 GlcNAc 的酶。此外,导致GlcNAc 前体降解的nagBB和murQ的缺失进一步改善了 GlcNAc 的合成。最好的工程菌株,枯草芽孢杆菌FMIP34 将摇瓶中的 GlcNAc 滴度从 11.5 g/L 增加到 26.1 g/L,并在 30-L 分批补料生物反应器中产生 87.5 g/L GlcNAc。我们的结果不仅首次阐明了枯草芽孢杆菌中的磷糖应激反应机制,而且展示了合理的代谢工程与对生理学和代谢的新见解的结合如何能够构建高效的微生物细胞工厂用于生产高价值化学品。
更新日期:2020-09-23
中文翻译:

枯草芽孢杆菌中磷糖应激反应的阐明指导了高 N-乙酰氨基葡萄糖生产的菌株工程。
枯草芽孢杆菌是营养食品工业生产的首选微生物宿主,也是合成功能性糖类(如N-乙酰氨基葡萄糖(GlcNAc))的有希望的候选者。以前,使用glmS核酶双调节工具构建了 GlcNAc 过量生产者B. subtilis SFMI 。在此,我们进一步设计以增强从葡萄糖到 GlcNAc 合成的碳通量。结果,增加的 GlcNAc 合成通量引发了磷糖应激反应,从而导致细胞生长异常。不幸的是,尚未阐明枯草芽孢杆菌中磷糖应激反应的机制. 为了揭示压力机制并克服其在生物生产中的负面影响,我们进行了比较转录组分析。结果表明细胞通过抑制葡萄糖输入来减缓葡萄糖利用并加速磷糖的分解代谢反应。为了验证这些结果,我们过度表达了磷酸酶 YwpJ,它减轻了磷酸糖压力,并允许我们鉴定负责从 GlcNAc 6-磷酸合成 GlcNAc 的酶。此外,导致GlcNAc 前体降解的nagBB和murQ的缺失进一步改善了 GlcNAc 的合成。最好的工程菌株,枯草芽孢杆菌FMIP34 将摇瓶中的 GlcNAc 滴度从 11.5 g/L 增加到 26.1 g/L,并在 30-L 分批补料生物反应器中产生 87.5 g/L GlcNAc。我们的结果不仅首次阐明了枯草芽孢杆菌中的磷糖应激反应机制,而且展示了合理的代谢工程与对生理学和代谢的新见解的结合如何能够构建高效的微生物细胞工厂用于生产高价值化学品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号