Water Research ( IF 11.4 ) Pub Date : 2020-09-23 , DOI: 10.1016/j.watres.2020.116447 Dan Li , Yin Zhong , Xifen Zhu , Heli Wang , Weiqiang Yang , Yirong Deng , Weilin Huang , Ping'an Peng
|
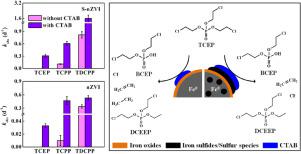
Chlorinated organophosphate esters (Cl-OPEs), e.g., tris(2-chloroethyl) phosphate (TCEP), tris(2-chloro-2-propyl) phosphate (TCPP) and tris(1,3-dichloro-2-propyl) phosphate (TDCPP), are widely used as additive flame retardants in commercial and building products. They have potential persistent organic pollutant properties and are frequently detected in various waters, especially in wastewaters. Nanoscale zerovalent iron (nZVI)-based method is an efficient reductive technology for treating waters polluted by halogenated organic pollutants (HOCs). Cetyltrimethylammonium bromide (CTAB) is a ubiquitous surfactant in wastewaters and can favorably affect the interaction between HOCs and nZVI. However, its effect on the Cl-OPEs removal by nZVI-based materials still remains unknown. Herein, the adsorption and degradation efficiencies of Cl-OPEs by nZVI and sulfidated nZVI (S-nZVI) in the presence or absence of CTAB were quantified based on the decreasing concentrations of Cl-OPEs in reaction systems. Our results showed that TDCPP and TCPP were adsorbed onto the nZVI or S-nZVI surface and subsequently degraded. In contrast, TCEP was just adsorbed onto the particle surface without further degradation. The addition of CTAB significantly enhanced the hydrophobic adsorption between Cl-OPEs and nZVI or S-nZVI, leading to increased degradation of Cl-OPEs (especially TCEP). CTAB adsorption isotherms indicated that S-nZVI had a higher adsorption capacity for CTAB than nZVI. The S-nZVI/CTAB composite exhibited a better performance than nZVI/CTAB composite. When S-nZVI was combined with 100.0 mg L−1 CTAB, 100% of TDCPP, TCPP and TCEP was degraded within 3 hours, 5 and 14 days, respectively. As the concentration of CTAB was increased up to 335.0 mg L−1, TCEP could be completely degraded within 3 days by S-nZVI. Five degradation products of TCEP were identified, of which O,O-di-(2-chloroethyl) O-ethyl phosphate (DCEEP) and ethane were reported for the first time. We propose that TCEP is dechlorinated by nZVI or S-nZVI through the electron attack at the ethyl-chlorine group to form bis(2-chloroethyl) phosphate, DCEEP, chloride, ethene and ethane, representing previously unknown degradation pathways.
中文翻译:

纳米级零价铁/鲸蜡基三甲基溴化铵复合物对氯化有机磷酸酯的还原降解:反应性,机理和新途径
氯化有机磷酸酯(Cl-OPEs),例如磷酸三(2-氯乙基)酯(TCEP),磷酸三(2-氯-2-丙基)酯(TCPP)和磷酸三(1,3-二氯-2-丙基)酯(TDCPP)被广泛用作商业和建筑产品中的添加剂阻燃剂。它们具有潜在的持久性有机污染物特性,并且经常在各种水中(尤其是废水中)被检测到。基于纳米零价铁(nZVI)的方法是一种有效的还原技术,用于处理被卤化有机污染物(HOC)污染的水。十六烷基三甲基溴化铵(CTAB)是废水中普遍存在的表面活性剂,可以有利地影响HOC与nZVI之间的相互作用。但是,其对基于nZVI的材料去除Cl-OPE的影响仍然未知。在这里 在存在或不存在CTAB的情况下,基于反应体系中Cl-OPEs浓度的降低,定量了nZVI和硫化nZVI(S-nZVI)对Cl-OPEs的吸附和降解效率。我们的结果表明,TDCPP和TCPP被吸附在nZVI或S-nZVI表面上,随后被降解。相反,TCEP只是吸附在颗粒表面而没有进一步降解。CTAB的添加显着增强了Cl-OPE与nZVI或S-nZVI之间的疏水吸附,从而导致Cl-OPE的降解增加(尤其是TCEP)。CTAB吸附等温线表明,S-nZVI对CTAB的吸附能力高于nZVI。S-nZVI / CTAB复合材料表现出比nZVI / CTAB复合材料更好的性能。当S-nZVI与100.0 mg L合并使用时 我们的结果表明,TDCPP和TCPP被吸附在nZVI或S-nZVI表面上,随后被降解。相反,TCEP只是吸附在颗粒表面而没有进一步降解。CTAB的添加显着增强了Cl-OPE与nZVI或S-nZVI之间的疏水吸附,从而导致Cl-OPE的降解增加(尤其是TCEP)。CTAB吸附等温线表明,S-nZVI对CTAB的吸附能力高于nZVI。S-nZVI / CTAB复合材料表现出比nZVI / CTAB复合材料更好的性能。当S-nZVI与100.0 mg L合并使用时 我们的结果表明,TDCPP和TCPP被吸附在nZVI或S-nZVI表面上,随后被降解。相反,TCEP只是吸附在颗粒表面而没有进一步降解。CTAB的添加显着增强了Cl-OPE与nZVI或S-nZVI之间的疏水吸附,从而导致Cl-OPE的降解增加(尤其是TCEP)。CTAB吸附等温线表明,S-nZVI对CTAB的吸附能力高于nZVI。S-nZVI / CTAB复合材料表现出比nZVI / CTAB复合材料更好的性能。当S-nZVI与100.0 mg L合并使用时 CTAB的添加显着增强了Cl-OPE与nZVI或S-nZVI之间的疏水吸附,从而导致Cl-OPE的降解增加(尤其是TCEP)。CTAB吸附等温线表明,S-nZVI对CTAB的吸附能力高于nZVI。S-nZVI / CTAB复合材料表现出比nZVI / CTAB复合材料更好的性能。当S-nZVI与100.0 mg L合并使用时 CTAB的添加显着增强了Cl-OPE与nZVI或S-nZVI之间的疏水吸附,从而导致Cl-OPE的降解增加(尤其是TCEP)。CTAB吸附等温线表明,S-nZVI对CTAB的吸附能力高于nZVI。S-nZVI / CTAB复合材料表现出比nZVI / CTAB复合材料更好的性能。当S-nZVI与100.0 mg L合并使用时-1 CTAB,TDCPP,TCPP和TCEP的100%分别在3小时,5和14天内降解。随着CTAB浓度增加至335.0 mg L -1,S-nZVI可以在3天内完全降解TCEP。鉴定了TCEP的五种降解产物,其中首次报道了O,O-二-(2-氯乙基)O-乙基磷酸酯(DCEEP)和乙烷。我们建议TCEP由nZVI或S-nZVI通过在乙基氯基团上的电子攻击而脱氯,以形成双(2-氯乙基)磷酸酯,DCEEP,氯离子,乙烯和乙烷,代表以前未知的降解途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号