Journal of Saudi Chemical Society ( IF 5.8 ) Pub Date : 2020-09-23 , DOI: 10.1016/j.jscs.2020.09.004 Usman Ghani , Shah Hussain , Noor-ul-Amin , Maria Imtiaz , Shahid Ali Khan
|
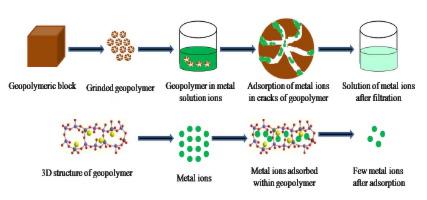
This study is focused on the investigation of low iron lateritic clay-based geopolymer as a potential adsorbent for the higher uptake of Ni(II) and Co(II) ions from aqueous solutions. BET analysis revealed that the sieved geopolymer sample (SGS) was characterized by 17.441 m2/g of surface area, 0.005 cm3/g of pore volume, and 13.549 Å of pore diameter. SEM investigation confirmed the presence of pores and cavities onto the surface of SGS. XRD analysis showed that the geopolymer is semi-crystalline in nature. It was found that the adsorption ability of SGS remained 520 mg/g for Ni(II) ions and 500 mg/g for Co(II) ions when 0.5 M solutions were stirred with SGS for 60 min. The temperature and pH of the solution were maintained at 60 °C and 7.0, respectively. The adsorption data of both heavy metal (HM) ions fitted best in the pseudo-second-order kinetic model. The low activation energy value i.e. 2.507 kJ/mol for Ni(II) ions and 2.286 kJ/mol for Co(II) ions confirmed adsorption is physisorption. Adsorption data were tested with Langmuir and Freundlich models, the data showed comparatively better fitting in the Freundlich model. The greater value of monolayer adsorption capacity (Xm) for Ni(II) ions was found 1.77 × 10−2 mol/g while for Co(II) ions it remained 1.69 × 10−2 mol/g confirming the better interaction of metal ions with the adsorbent surface. Negative values of ΔG° confirmed the spontaneity of the process while the positive value of ΔS° showed the randomness of adsorbate particles. The positive value of ΔH° showed that the adsorption process remained endothermic for both HM ions. The experimental results confirmed the ability of laterite clay-based geopolymer for better removal of HM ions and hence can be employed for the wastewater treatment processes at low-cost adsorbent.
中文翻译:

红土粘土基地质聚合物作为从水溶液中去除重金属的潜在吸附剂
这项研究的重点是研究低铁红土粘土基地质聚合物作为潜在的吸附剂,以从水溶液中吸收更高的Ni(II)和Co(II)离子。BET分析表明,筛分的地质聚合物样品(SGS)的特征是表面积为17.441 m 2 /g,0.005 cm 3/ g的孔容和13.549Å的孔径。SEM研究证实SGS表面上存在孔和腔。XRD分析表明该地质聚合物本质上是半结晶的。发现将0.5 M溶液与SGS搅拌60分钟后,SGS对Ni(II)离子的吸附能力保持为520 mg / g,对Co(II)离子的吸附能力保持为500 mg / g。溶液的温度和pH分别保持在60℃和7.0。两种重金属离子的吸附数据最适合拟二级动力学模型。低活化能值,即Ni(II)离子为2.507 kJ / mol,Co(II)离子为2.286 kJ / mol,证实了吸附是物理吸附。用Langmuir和Freundlich模型测试了吸附数据,数据显示在Freundlich模型中具有相对较好的拟合度。-2 mol / g,而对于Co(II)离子则保持1.69×10 -2 mol / g,这证实了金属离子与吸附剂表面的相互作用更好。ΔG°的负值确认了过程的自发性,而ΔS°的正值表明了被吸附物颗粒的随机性。ΔH°的正值表明两种HM离子的吸附过程均保持吸热。实验结果证实了红土粘土基地质聚合物能够更好地除去HM离子,因此可用于低成本吸附剂的废水处理过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号