Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-09-23 , DOI: 10.1016/j.molliq.2020.114408 Jing Ma , Yutong Wang , Mingxuan Zhu , Xueqing Yang , Baohe Wang
|
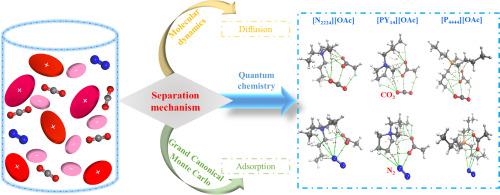
Based on the favorable absorption performance of acetate anion-based ionic liquids (AcILs) for CO2, which has been confirmed in experiments, three representative AcILs, i.e., triethylbutylammonium acetate ([N2224][OAc]), 1-butyl-1-methylpyrrolidinium acetate ([PY14][OAc]) and tetrabutylphosphine acetate ([P4444][OAc]), were investigated for the separation of CO2/N2 from flue gas, and combined quantum chemistry (QC), molecular dynamics (MD) and Grand Canonical Monte Carlo (GCMC) methods, the separation mechanism of AcILs on CO2/N2 was revealed from a microscopic perspective. Herein, stable configurations for AcILs-gas molecules were determined by electrostatic potential (ESP) analysis and interaction energies calculation. The interaction nature of AcILs with gas molecules was characterized by atoms in molecules (AIM) theory, which indicated that both anions and cations interacted with gas molecules, and the interaction of AcILs with CO2 was stronger than those with N2 due to the formation of weak hydrogen bond (H-bond) between them. Meanwhile, MD results suggested that the interaction between AcILs and CO2 was dominated by [OAc]−, and cations also played important roles. The effect of diffusion on separation mechanism was also further illustrated, and large self-diffusion coefficients (Ds) of N2 over CO2 were identified. Moreover, the adsorption isotherms of CO2/N2 molecules in three AcILs at different pressures were calculated using GCMC method. The results showed that all AcILs exhibited high adsorption selectivity for CO2, and [N2224][OAc] had the strongest solubility among them, which were in accordance with the QC analyses results. Hopefully, this work could contribute to better understand the microscopic interaction mechanisms between AcILs and CO2/N2, give an intuitive explanation for their solubility differences, and ultimately provide theoretical guidance for the screening of CO2/N2 separation absorbents.
中文翻译:

深入了解乙酸根阴离子基离子液体在CO 2和N 2上的分离机理:多尺度模拟研究
在实验中已经证实,基于乙酸根阴离子基离子液体(AcILs)对CO 2的良好吸收性能,三种代表性的AcILs,即三乙基丁基乙酸铵([N 2224 ] [OAc]),1-丁基-1研究了乙酸-甲基吡咯烷鎓乙酸盐([PY 14 ] [OAc])和乙酸四丁基膦乙酸酯([P 4444 ] [OAc])的分离作用,从烟气中分离出CO 2 / N 2,并结合量子化学(QC),分子动力学(MD)和大正则蒙特卡罗(GCMC)方法,ACIL在CO 2 / N 2上的分离机理是从微观角度揭示的。在此,通过静电势(ESP)分析和相互作用能计算来确定AcILs-气体分子的稳定构型。AcILs与气体分子的相互作用本质是通过分子中的原子(AIM)理论来表征的,这表明阴离子和阳离子都与气体分子相互作用,并且由于形成,AcILs与CO 2的相互作用比与N 2的相互作用更强。它们之间的弱氢键(氢键)的关系。同时,MD结果表明[OAc] -决定了ACIL与CO 2之间的相互作用。,阳离子也起重要作用。还进一步说明了扩散对分离机理的影响,并确定了N 2在CO 2上的大自扩散系数(D s)。此外,采用GCMC法计算了三种AcIL在不同压力下的CO 2 / N 2分子吸附等温线。结果表明,所有AcIL对CO 2均具有较高的吸附选择性,其中[N 2224 ] [OAc]的溶解度最强,这与QC分析结果一致。希望这项工作可以有助于更好地了解AcIL和CO 2之间的微观相互作用机理/ N 2给出它们溶解度差异的直观解释,并最终为筛选CO 2 / N 2分离吸收剂提供理论指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号