Cell Systems ( IF 9.0 ) Pub Date : 2020-09-23 , DOI: 10.1016/j.cels.2020.08.019 Mickael Meyer 1 , Agnès Paquet 2 , Marie-Jeanne Arguel 2 , Ludovic Peyre 1 , Luis C Gomes-Pereira 3 , Kevin Lebrigand 2 , Baharia Mograbi 1 , Patrick Brest 1 , Rainer Waldmann 2 , Pascal Barbry 2 , Paul Hofman 1 , Jérémie Roux 1
|
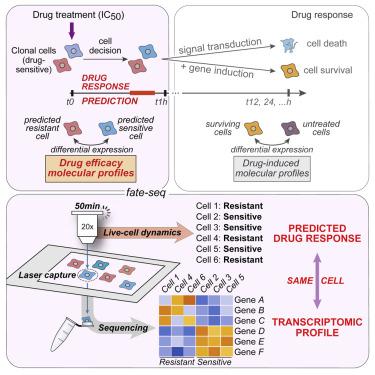
Non-genetic heterogeneity observed in clonal cell populations is an immediate cause of drug resistance that remains challenging to profile because of its transient nature. Here, we coupled three single-cell technologies to link the predicted drug response of a cell to its own genome-wide transcriptomic profile. As a proof of principle, we analyzed the response to tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) in HeLa cells to demonstrate that cell dynamics can discriminate the transient transcriptional states at the origin of cell decisions such as sensitivity and resistance. Our same-cell approach, named fate-seq, can reveal the molecular factors regulating the efficacy of a drug in clonal cells, providing therapeutic targets of non-genetic drug resistance otherwise confounded in gene expression noise. A record of this paper’s transparent peer review process is included in the Supplemental Information.
中文翻译:

使用预测细胞动力学通过单细胞功能基因组学方法分析癌症耐药性的非遗传起源
在克隆细胞群中观察到的非遗传异质性是耐药性的直接原因,由于其短暂的性质,耐药性仍然具有挑战性。在这里,我们结合了三种单细胞技术,将细胞的预测药物反应与其自身的全基因组转录组谱联系起来。作为原理证明,我们分析了 HeLa 细胞对肿瘤坏死因子相关凋亡诱导配体 (TRAIL) 的反应,以证明细胞动力学可以区分细胞决定起源时的瞬时转录状态,例如敏感性和抗性. 我们的同细胞方法,命名为命运序列,可以揭示在克隆细胞中调节药物功效的分子因素,提供非遗传耐药性的治疗靶点,否则会被基因表达噪声混淆。











































 京公网安备 11010802027423号
京公网安备 11010802027423号