当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of CO2 pressure, temperature, and brine composition on the interlayer spacing of Na-rich and K-exchanged montmorillonite
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.clay.2020.105819 Paolo Andre Benavides , Jacqueline Kowalik , Stephen Guggenheim , August F. Koster van Groos
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.clay.2020.105819 Paolo Andre Benavides , Jacqueline Kowalik , Stephen Guggenheim , August F. Koster van Groos
|
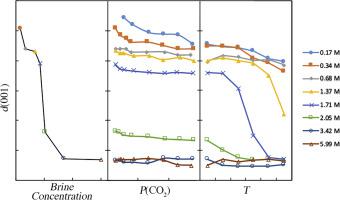
Abstract One of the more important strategies to curb the increase of anthropogenic carbon dioxide (CO2) in the atmosphere is to inject large amounts of supercritical CO2 (scCO2) into the sandstones of deep saline sandstone-shale sequences, where the shale layers act as impermeable seals. An important clay mineral constituent in these sequences is smectite, which exhibits significant volume changes in response to the activity of H2O [a(H2O)]. The present study investigates the effect of a(H2O) on the molar volume of smectite in a brine by varying temperature (T), CO2 pressure [P(CO2)], and brine concentration. In addition, because inert gases do not interact with smectite, a series of experiments using He as a pressure medium at similar P-T conditions were made for comparison. A high-pressure environmental chamber (HPEC) was used to investigate the effects of T, P(CO2), P(He), and brine composition on the d(001) of montmorillonite (Clay Minerals Society Source Clay SWy2) using X-ray diffraction. Measurements were performed using a Na-rich montmorillonite (Na-SWy2) and a K-exchanged montmorillonite (K-exchanged SWy2) at P(CO2) and P(He) in intervals of 100 bars to 500 bars and T in intervals of 25 °C to 150 °C. Eight NaCl brines with concentrations ranging from 0.17 M to saturation and a select series of KCl brines were used. Increasing the NaCl brine concentrations from 0.34 M to saturation at P(CO2) = 1 bar and T ~ 33 °C resulted in a substantial decrease of d(001) of Na-SWy2 from 20.1 to 15.7 A (25%). In contrast, increasing P(CO2) from 55 to 500 bars at T ~ 33 °C and a brine concentration of 0.17 M, resulted in a decrease in d(001) from 20.4 to 19.6 A (4%). Using a saturated brine (5.99 M), increasing P(CO2) from 1 bar to 500 bars did not cause a significant decrease in d(001). Similar trends were observed when T was increased from ~33 to 150 °C. At 0.17 M brine concentration, d(001) decreased from 19.6 to 19.0 A (3%). At 1.71 M, d(001) decreased from 18.6 to 15.7 A (17%) with T = 50 to 150 °C. Experiments using P(He) as the pressure medium showed identical results with the experiments with CO2, indicating that at the P-T range of these experiments and with excess water, CO2 does not enter the interlayer of these clay minerals. In the experiments with K-exchanged SWy2, d(001) measurements were not possible because K-exchanged SWy2 lacks periodicity and different hydration states are randomly distributed in the structure. The results show that both T and brine concentration have a major effect on d(001) because of the loss or addition of interlayer H2O, i.e. change in molar volume in Na-SWy2, whereas P(CO2) is less important. Smectite in reservoir sandstones may be present as a coating of mineral grains. An increase in a(H2O) can, therefore, decrease the permeability of the reservoir sandstone because of the expansion of smectite. Similarly, an increase in a(H2O) can enhance the sealing property of the confining shales, because these can contain a significant amount of smectite. In contrast, contraction of Na-SWy2 may occur when a(H2O) decreases. The contraction can cause shrinkage of the shale and produce secondary fractures that may form conduits for CO2 to escape.
中文翻译:

CO2压力、温度和卤水成分对富钠和钾交换蒙脱石层间距的影响
摘要 抑制大气中人为二氧化碳 (CO2) 增加的更重要策略之一是将大量超临界 CO2 (scCO2) 注入深盐水砂岩-页岩层序的砂岩中,其中页岩层起到不渗透作用。密封件。在这些序列中,一种重要的粘土矿物成分是蒙脱石,它表现出显着的体积变化以响应 H2O [a(H2O)] 的活性。本研究通过改变温度 (T)、CO2 压力 [P(CO2)] 和盐水浓度,研究了 a(H2O) 对盐水中蒙脱石摩尔体积的影响。此外,由于惰性气体不与蒙脱石相互作用,因此在类似的 PT 条件下进行了一系列使用 He 作为压力介质的实验进行比较。高压环境室 (HPEC) 用于研究 T、P(CO2)、P(He) 和盐水成分对蒙脱石(Clay Minerals Society Source Clay SWy2)的 d(001) 的影响,使用 X-射线衍射。使用富钠蒙脱石 (Na-SWy2) 和钾交换蒙脱石 (K-交换 SWy2) 在 P(CO2) 和 P(He) 下以 100 巴至 500 巴的间隔和 T 以 25 巴的间隔进行测量°C 至 150 °C。使用了八种浓度范围从 0.17 M 到饱和的 NaCl 盐水和一系列精选的 KCl 盐水。在 P(CO2) = 1 bar 和 T ~ 33 °C 时,将 NaCl 盐水浓度从 0.34 M 增加到饱和,导致 Na-SWy2 的 d(001) 从 20.1 A 大幅降低至 15.7 A (25%)。相比之下,在 T ~ 33 °C 和 0.17 M 的盐水浓度下,将 P(CO2) 从 55 bar 增加到 500 bar,导致 d(001) 从 20.4 A 降低到 19.6 A (4%)。使用饱和盐水 (5.99 M),将 P(CO2) 从 1 bar 增加到 500 bar 不会导致 d(001) 显着降低。当 T 从~33°C 增加到 150°C 时,观察到类似的趋势。在 0.17 M 盐水浓度下,d(001) 从 19.6 A 降至 19.0 A (3%)。在 1.71 M 时,d(001) 从 18.6 A 降至 15.7 A (17%),T = 50 至 150 °C。使用 P(He) 作为压力介质的实验显示出与使用 CO2 的实验相同的结果,表明在这些实验的 PT 范围和过量的水下,CO2 不会进入这些粘土矿物的夹层。在 K 交换 SWy2 的实验中,d(001) 测量是不可能的,因为 K 交换 SWy2 缺乏周期性,并且不同的水合状态随机分布在结构中。结果表明,由于层间 H2O 的损失或添加,即 Na-SWy2 中摩尔体积的变化,T 和盐水浓度对 d(001) 有主要影响,而 P(CO2) 不太重要。储层砂岩中的蒙脱石可能作为矿物颗粒的涂层存在。因此,a(H2O) 的增加会由于蒙脱石的膨胀而降低储层砂岩的渗透率。类似地,a(H2O) 的增加可以增强封闭页岩的密封性能,因为这些页岩可能含有大量的蒙脱石。相反,当 a(H2O) 减少时,Na-SWy2 可能会发生收缩。收缩会导致页岩收缩并产生可能形成二氧化碳逃逸管道的次生裂缝。
更新日期:2020-11-01
中文翻译:

CO2压力、温度和卤水成分对富钠和钾交换蒙脱石层间距的影响
摘要 抑制大气中人为二氧化碳 (CO2) 增加的更重要策略之一是将大量超临界 CO2 (scCO2) 注入深盐水砂岩-页岩层序的砂岩中,其中页岩层起到不渗透作用。密封件。在这些序列中,一种重要的粘土矿物成分是蒙脱石,它表现出显着的体积变化以响应 H2O [a(H2O)] 的活性。本研究通过改变温度 (T)、CO2 压力 [P(CO2)] 和盐水浓度,研究了 a(H2O) 对盐水中蒙脱石摩尔体积的影响。此外,由于惰性气体不与蒙脱石相互作用,因此在类似的 PT 条件下进行了一系列使用 He 作为压力介质的实验进行比较。高压环境室 (HPEC) 用于研究 T、P(CO2)、P(He) 和盐水成分对蒙脱石(Clay Minerals Society Source Clay SWy2)的 d(001) 的影响,使用 X-射线衍射。使用富钠蒙脱石 (Na-SWy2) 和钾交换蒙脱石 (K-交换 SWy2) 在 P(CO2) 和 P(He) 下以 100 巴至 500 巴的间隔和 T 以 25 巴的间隔进行测量°C 至 150 °C。使用了八种浓度范围从 0.17 M 到饱和的 NaCl 盐水和一系列精选的 KCl 盐水。在 P(CO2) = 1 bar 和 T ~ 33 °C 时,将 NaCl 盐水浓度从 0.34 M 增加到饱和,导致 Na-SWy2 的 d(001) 从 20.1 A 大幅降低至 15.7 A (25%)。相比之下,在 T ~ 33 °C 和 0.17 M 的盐水浓度下,将 P(CO2) 从 55 bar 增加到 500 bar,导致 d(001) 从 20.4 A 降低到 19.6 A (4%)。使用饱和盐水 (5.99 M),将 P(CO2) 从 1 bar 增加到 500 bar 不会导致 d(001) 显着降低。当 T 从~33°C 增加到 150°C 时,观察到类似的趋势。在 0.17 M 盐水浓度下,d(001) 从 19.6 A 降至 19.0 A (3%)。在 1.71 M 时,d(001) 从 18.6 A 降至 15.7 A (17%),T = 50 至 150 °C。使用 P(He) 作为压力介质的实验显示出与使用 CO2 的实验相同的结果,表明在这些实验的 PT 范围和过量的水下,CO2 不会进入这些粘土矿物的夹层。在 K 交换 SWy2 的实验中,d(001) 测量是不可能的,因为 K 交换 SWy2 缺乏周期性,并且不同的水合状态随机分布在结构中。结果表明,由于层间 H2O 的损失或添加,即 Na-SWy2 中摩尔体积的变化,T 和盐水浓度对 d(001) 有主要影响,而 P(CO2) 不太重要。储层砂岩中的蒙脱石可能作为矿物颗粒的涂层存在。因此,a(H2O) 的增加会由于蒙脱石的膨胀而降低储层砂岩的渗透率。类似地,a(H2O) 的增加可以增强封闭页岩的密封性能,因为这些页岩可能含有大量的蒙脱石。相反,当 a(H2O) 减少时,Na-SWy2 可能会发生收缩。收缩会导致页岩收缩并产生可能形成二氧化碳逃逸管道的次生裂缝。











































 京公网安备 11010802027423号
京公网安备 11010802027423号