Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-09-22 , DOI: 10.1016/j.apcatb.2020.119543 Xueyan Chen , Honghong Wang , Min Chen , Xiaoxiao Qin , Hong He , Changbin Zhang
|
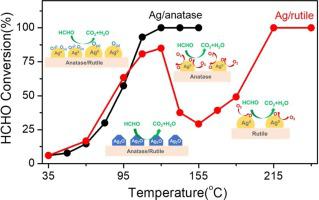
Ag-based catalysts are shown to be efficient for formaldehyde (HCHO) oxidation at low temperature, while the role of active Ag species in this reaction remains a controversial issue. In this work, we prepared Ag/TiO2 catalysts with TiO2 of different crystal structures (anatase or rutile) as supports and tested their performances in HCHO oxidation. We observed that the crystal structure of the TiO2 support had a significant influence on the activity of the Ag/TiO2 catalysts, and Ag/A (anatase) exhibited dramatically superior activity compared with Ag/R (rutile). More interestingly, we observed a clear inflection point in the HCHO conversion curve for the Ag/R catalyst. Combining the results of BET, XRD, TEM, XAFS, H2-TPR characterization and DFT calculations, we show that HCHO oxidation on Ag/TiO2 catalysts is a co-function process of multiple active sites including surface oxygen, Ag2O, and metallic Ag species. The low-temperature non-renewable surface oxygen and Ag2O species mainly contribute to HCHO oxidation in the low-temperature range, while metallic Ag species are primarily responsible for the reaction at high temperature since metallic Ag is capable of activation of oxygen at high temperature. The relative contents of surface oxygen, Ag2O and metallic Ag species are different on the Ag/A and Ag/R catalysts. The dispersion degree of metallic Ag species also differs on the Ag/A and Ag/R, therefore their capacities for oxygen activation are quite dissimilar. The above factors combine to result in distinct catalytic behaviors for Ag/A and Ag/R in HCHO oxidation, as well as the appearance of an inflection point.
中文翻译:

Ag / TiO 2上多个活性位点对甲醛氧化的协同作用机理
银基催化剂在低温下对甲醛(HCHO)氧化有效,而活性银在该反应中的作用仍然是一个有争议的问题。在这项工作中,我们制备了具有不同晶体结构(锐钛矿或金红石)的TiO 2作为载体的Ag / TiO 2催化剂,并测试了它们在HCHO氧化中的性能。我们观察到,TiO 2载体的晶体结构对Ag / TiO 2催化剂的活性有显着影响,与Ag / R(金红石)相比,Ag / A(锐钛矿)显示出显着优越的活性。更有趣的是,我们在Ag / R催化剂的HCHO转化曲线中观察到明显的拐点。结合BET,XRD,TEM,XAFS,H 2的结果-TPR表征和DFT计算,我们表明Ag / TiO 2催化剂上的HCHO氧化是包括表面氧,Ag 2 O和金属Ag物种在内的多个活性位的协同过程。低温不可再生表面氧和Ag 2 O物种在低温范围内主要导致HCHO氧化,而金属Ag物种主要负责高温下的反应,因为金属Ag能够在高温下活化氧温度。表面氧的相对含量,Ag 2Ag / A和Ag / R催化剂上的O和金属Ag种类不同。金属Ag的分散度在Ag / A和Ag / R上也有所不同,因此它们的氧活化能力非常不同。以上因素共同导致了HCHO氧化过程中Ag / A和Ag / R的独特催化行为以及拐点的出现。









































 京公网安备 11010802027423号
京公网安备 11010802027423号