当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of 1,2,4-triazole derivatives by oxidation of 4-phenyl-1-pivaloylsemicarbazide
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-09-23 , DOI: 10.1007/s10593-020-02768-4 Boris А. Gostevskii , Aleksander I. Albanov , Aleksander V. Vashchenko , Nataliya F. Lazareva
中文翻译:

通过4-苯基-1-新戊酰氨基脲的氧化形成1,2,4-三唑衍生物
更新日期:2020-09-23
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-09-23 , DOI: 10.1007/s10593-020-02768-4 Boris А. Gostevskii , Aleksander I. Albanov , Aleksander V. Vashchenko , Nataliya F. Lazareva
|
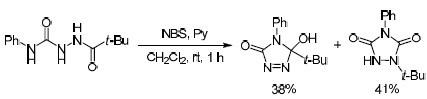
Oxidation of 4-phenyl-1-pivaloylsemicarbazide leads to the formation of two structural isomers: 5-tert-butyl-5-hydroxy-4-phenyl-4,5-dihydro-3H-1,2,4-triazol-3-one and 1-tert-butyl-4-phenyl-1,2,4-triazolidine-3,5-dione. Isomerization of 5-tert-butyl-5-hydroxy-4-phenyl-4,5-dihydro-3H-1,2,4-triazol-3-one to 1-tert-butyl-4-phenyl-1,2,4-triazolidine-3,5-dione proceeds upon heating and is accompanied by the migration of the tert-butyl group from the carbon atom to the nitrogen atom.
中文翻译:

通过4-苯基-1-新戊酰氨基脲的氧化形成1,2,4-三唑衍生物
4-苯基-1-新戊酰氨基脲的氧化导致形成两种结构异构体:5-叔丁基-5-羟基-4-苯基-4,5-二氢-3 H -1,2,4-三唑-3 -一和1-叔丁基-4-苯基-1,2,4-三唑烷-3,5-二酮。5-叔丁基-5-羟基-4-苯基-4,5-二氢-3 H -1,2,4-三唑-3-酮异构化为1-叔丁基-4-苯基-1,2 1,4-三唑烷-3,5-二酮在加热时进行,并伴随着叔丁基从碳原子向氮原子的迁移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号