Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH dependency of the structural and photophysical properties of the atypical 2′,3-dihydroxyflavone
RSC Advances ( IF 3.9 ) Pub Date : 2020-9-22 , DOI: 10.1039/d0ra06833k Luc Labarrière 1 , Aurélien Moncomble 1 , Jean-Paul Cornard 1
RSC Advances ( IF 3.9 ) Pub Date : 2020-9-22 , DOI: 10.1039/d0ra06833k Luc Labarrière 1 , Aurélien Moncomble 1 , Jean-Paul Cornard 1
Affiliation
|
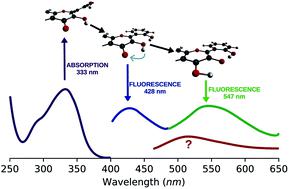
2′,3-Dihydroxyflavone (2′3HF) is a natural flavonol that has barely ever been studied, however the scarce studies of its physico-chemical properties have highlighted its atypical behaviour. We present a structural and spectral study of 2′3HF, performed using UV-visible absorption and fluorescence spectroscopies, coupled with DFT and TD-DFT calculations. Although its structure is close to that of 3-hydroxyflavone, 2′3HF shows a much lower pKa value. We show that the origin of this particularity is the substitution by a hydroxyl group on position 2′, that induces a stronger inter-ring interaction weakening the bonding of the proton at position 3. The main absorption band of the is red-shifted upon deprotonation. The remaining proton is highly bonded in between oxygen atoms 3 and 2′, making the second deprotonation unattainable in methanol. The neutral form can undergo an excited-state intramolecular proton transfer to emit dual fluorescence by the normal and tautomer forms. We suggested five geometries to be the sources of the emission bands, and showed that the energy barriers to interconversions were almost null. The anion is also fluorescent. The Stokes shifts for the neutral normal and anion species are extremely high, that can be explained by the conformational rearrangement, as the species go from twisted in the ground-state, to planar in the excited-state. Finally, another emission band is evidenced when exciting in the vicinity of the absorption maximum of the anion species in acidic medium. We suggest an aggregate with the solvent to be the origin of the emission.
中文翻译:

非典型 2',3-二羟基黄酮的结构和光物理性质的 pH 依赖性
2',3-二羟基黄酮 (2'3HF) 是一种天然黄酮醇,几乎没有被研究过,但对其物理化学性质的研究很少,突出了它的非典型行为。我们提出了 2'3HF 的结构和光谱研究,该研究使用紫外-可见吸收和荧光光谱以及 DFT 和 TD-DFT 计算进行。虽然其结构接近于 3-羟基黄酮,但 2'3HF 的 p K a低得多价值。我们表明,这种特殊性的起源是位置 2' 上的羟基取代,这会引起更强的环间相互作用,从而削弱质子在位置 3 的键合。去质子化时 的主要吸收带发生红移. 剩余的质子高度键合在氧原子 3 和 2' 之间,使得在甲醇中无法进行第二次去质子化。中性形式可以经历激发态分子内质子转移以通过正常和互变异构形式发射双重荧光。我们建议将五种几何形状作为发射带的来源,并表明相互转换的能量障碍几乎为零。阴离子也是荧光的。中性正态和阴离子物种的斯托克斯位移非常高,这可以通过构象重排来解释,随着物种从基态的扭曲转变为激发态的平面。最后,当在酸性介质中阴离子物质的吸收最大值附近激发时,证明了另一个发射带。我们建议将带有溶剂的聚合体作为排放源。
更新日期:2020-09-22
中文翻译:

非典型 2',3-二羟基黄酮的结构和光物理性质的 pH 依赖性
2',3-二羟基黄酮 (2'3HF) 是一种天然黄酮醇,几乎没有被研究过,但对其物理化学性质的研究很少,突出了它的非典型行为。我们提出了 2'3HF 的结构和光谱研究,该研究使用紫外-可见吸收和荧光光谱以及 DFT 和 TD-DFT 计算进行。虽然其结构接近于 3-羟基黄酮,但 2'3HF 的 p K a低得多价值。我们表明,这种特殊性的起源是位置 2' 上的羟基取代,这会引起更强的环间相互作用,从而削弱质子在位置 3 的键合。去质子化时 的主要吸收带发生红移. 剩余的质子高度键合在氧原子 3 和 2' 之间,使得在甲醇中无法进行第二次去质子化。中性形式可以经历激发态分子内质子转移以通过正常和互变异构形式发射双重荧光。我们建议将五种几何形状作为发射带的来源,并表明相互转换的能量障碍几乎为零。阴离子也是荧光的。中性正态和阴离子物种的斯托克斯位移非常高,这可以通过构象重排来解释,随着物种从基态的扭曲转变为激发态的平面。最后,当在酸性介质中阴离子物质的吸收最大值附近激发时,证明了另一个发射带。我们建议将带有溶剂的聚合体作为排放源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号