当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of thermodynamic functions and structural parameters of NpO2+ lactate complexes
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-09-22 , DOI: 10.1039/d0nj04291a M. M. Maiwald 1, 2, 3, 4, 5 , K. Müller 5, 6, 7, 8 , K. Heim 5, 6, 7, 8 , M. Trumm 5, 9, 10, 11 , N. L. Banik 5, 12, 13, 14, 15 , J. Rothe 5, 9, 10, 11 , K. Dardenne 5, 9, 10, 11 , A. Skerencak-Frech 5, 9, 10, 11 , P. J. Panak 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-09-22 , DOI: 10.1039/d0nj04291a M. M. Maiwald 1, 2, 3, 4, 5 , K. Müller 5, 6, 7, 8 , K. Heim 5, 6, 7, 8 , M. Trumm 5, 9, 10, 11 , N. L. Banik 5, 12, 13, 14, 15 , J. Rothe 5, 9, 10, 11 , K. Dardenne 5, 9, 10, 11 , A. Skerencak-Frech 5, 9, 10, 11 , P. J. Panak 1, 2, 3, 4, 5
Affiliation
|
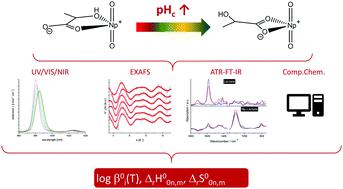
The complexation of NpO2+ with lactate in aqueous solution is studied as a function of the total ligand concentration ([Lac−]total), ionic strength (Im = 0.5–4.0 mol kg−1 Na+(Cl−/ClO4−)) and temperature (T = 20–85 °C) by Vis/NIR absorption spectroscopy. The formation of two NpO2+ lactate species with the stoichiometry NpO2(Lac)n1−n (n = 1, 2) is observed at the studied experimental conditions. The temperature dependent conditional stability constants log βj′(T) at different ionic strengths are calculated with the law of mass action. The conditional data are extrapolated to IUPAC reference state conditions (Im = 0) with the specific ion interaction theory (SIT). With increasing temperature up to 85 °C log β01(20 °C) = 1.92 ± 0.14 decreases by 0.12 and log β02(20 °C) = 2.10 ± 0.13 decreases by 0.17. The thermodynamic stability constants correlate linearly with the reciprocal temperature according to the integrated Van’t Hoff equation. Thus, linear regression analyses yield the standard reaction enthalpy ΔrH0 and entropy ΔrS0 for the complexation reactions. In addition, the sum of the SIT specific binary ion–ion interaction coefficients Δεj,k(T) of the complexation reactions are determined by variation of the ionic strength. Structural parameters of the formed complex species and the coordination mode of lactate towards the NpO2+ ion are investigated as a function of pHc by extended X-ray absorption fine structure spectroscopy (EXAFS) and attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FT IR). The results show, that the coordination mode of lactate changes from end-on (coordination via only the COO− group) to side-on (formation of chelate rings involving the OH-group) with increasing pHc. The experiments are supported by quantum chemical calculations.
中文翻译:

NpO2 +乳酸配合物的热力学函数和结构参数的确定
NPO的络合2 +在水溶液中与乳酸研究为总配体浓度的函数([紫胶- ]总),离子强度(我米= 0.5-4.0摩尔千克-1的Na +(CL - / CLO 4 −)和温度(T = 20–85°C),采用Vis / NIR吸收光谱法。化学计量比为NpO 2(Lac)n 1- n(n的两种NpO 2 +乳酸物质的形成= 1,2)在研究的实验条件下观察到。取决于条件稳定性常数日志温度 β Ĵ '(Ť在不同离子强度)与质量作用定律计算。使用特定离子相互作用理论(SIT)将条件数据外推至IUPAC参考状态条件(I m = 0)。随着温度的升高至85℃,日志 β 0 1(20℃)0.12 1.92 =±0.14减少和日志 β 0 2(20°C)= 2.10±0.13降低0.17。根据积分的Van't Hoff方程,热力学稳定性常数与倒数温度线性相关。因此,线性回归分析得出络合反应的标准反应焓Δr H 0和熵Δr S 0。此外,SIT特定的二进制离子-离子相互作用系数的总和Δ ε Ĵ,ķ(Ť的络合反应的)通过的离子强度的变化来确定。形成的复杂物种的结构参数和乳酸对NpO 2 +的配位模式通过扩展X射线吸收精细结构光谱(EXAFS)和衰减全反射傅里叶变换红外光谱(ATR-FT IR)研究了离子与pH c的关系。结果表明,即乳酸的配位模式从端上(协调改变通过仅COO -基团)随着pH增加至侧面上(形成涉及OH基团螯合环的)Ç。实验得到了量子化学计算的支持。
更新日期:2020-10-15
中文翻译:

NpO2 +乳酸配合物的热力学函数和结构参数的确定
NPO的络合2 +在水溶液中与乳酸研究为总配体浓度的函数([紫胶- ]总),离子强度(我米= 0.5-4.0摩尔千克-1的Na +(CL - / CLO 4 −)和温度(T = 20–85°C),采用Vis / NIR吸收光谱法。化学计量比为NpO 2(Lac)n 1- n(n的两种NpO 2 +乳酸物质的形成= 1,2)在研究的实验条件下观察到。取决于条件稳定性常数日志温度 β Ĵ '(Ť在不同离子强度)与质量作用定律计算。使用特定离子相互作用理论(SIT)将条件数据外推至IUPAC参考状态条件(I m = 0)。随着温度的升高至85℃,日志 β 0 1(20℃)0.12 1.92 =±0.14减少和日志 β 0 2(20°C)= 2.10±0.13降低0.17。根据积分的Van't Hoff方程,热力学稳定性常数与倒数温度线性相关。因此,线性回归分析得出络合反应的标准反应焓Δr H 0和熵Δr S 0。此外,SIT特定的二进制离子-离子相互作用系数的总和Δ ε Ĵ,ķ(Ť的络合反应的)通过的离子强度的变化来确定。形成的复杂物种的结构参数和乳酸对NpO 2 +的配位模式通过扩展X射线吸收精细结构光谱(EXAFS)和衰减全反射傅里叶变换红外光谱(ATR-FT IR)研究了离子与pH c的关系。结果表明,即乳酸的配位模式从端上(协调改变通过仅COO -基团)随着pH增加至侧面上(形成涉及OH基团螯合环的)Ç。实验得到了量子化学计算的支持。










































 京公网安备 11010802027423号
京公网安备 11010802027423号