当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reductive transformations of dichloroacetamide safeners: effects of agrochemical co-formulants and iron oxide + manganese oxide binary-mineral systems.
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-09-22 , DOI: 10.1039/d0em00331j Allison N Ricko 1 , Andrew W Psoras , John D Sivey
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-09-22 , DOI: 10.1039/d0em00331j Allison N Ricko 1 , Andrew W Psoras , John D Sivey
Affiliation
|
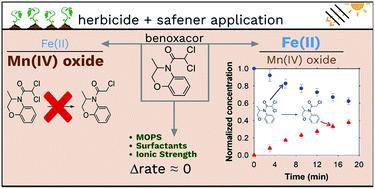
The toxic effects of herbicides are often incompletely selective and can harm crops. Safeners are “inert” ingredients commonly added to herbicide formulations to protect crops from herbicide-induced injury. Dichloroacetamide safeners have been previously shown to undergo reductive dechlorination in anaerobic abiotic systems containing an iron (hydr)oxide mineral (goethite or hematite) amended with Fe(II). Manganese oxides (e.g., birnessite) are important redox-active species that frequently co-occur with iron (hydr)oxides, yet studies examining the effects of more than one mineral on transformations of environmental contaminants are rare. Herein, we investigate the reactivity of dichloroacetamide safeners benoxacor, furilazole, and dichlormid in binary-mineral, anaerobic systems containing Fe(II)-amended hematite and birnessite. As the molar ratio of Fe(II)-to-Mn(IV) oxide increased, the transformation rate of benoxacor and furilazole increased. The safener dichlormid did not transform appreciably over the sampling period (6 hours). The concentration of pH buffer ([MOPS] = 10–50 mM), ionic strength ([NaCl] = 10–200 mM), and order of solute addition (e.g., safener followed by Fe(II) or vice versa) do not appreciably affect transformation rates of the examined dichloroacetamide safeners in Fe(II) + hematite slurries. The presence of agrochemical co-formulants, including the herbicide S-metolachlor and three surfactants, in solutions containing Cr(H2O)62+ (as a model homogeneous reductant) also did not substantially influence rates of safener transformation. This study is among the first to examine laboratory systems of intermediate complexity (e.g., systems containing mixtures of agrochemical co-formulants or mineral phases) when assessing the environmental fate of emerging contaminants such as dichloroacetamide safeners.
中文翻译:

二氯乙酰胺安全剂的还原性转化:农药共制剂和氧化铁+氧化锰二元矿物系统的影响。
除草剂的毒性作用通常选择不完全,会损害农作物。安全剂是通常添加到除草剂配方中的“惰性”成分,可保护农作物免受除草剂引起的伤害。先前已证明二氯乙酰胺安全剂在厌氧的非生物系统中进行还原脱氯,该系统含有铁(氢)氧化物矿物质(针铁矿或赤铁矿),并经Fe(II)改性。锰氧化物(例如,水钠锰矿)是重要的氧化还原活性物种,经常与氧化铁(氢氧化物)同时存在,但很少有研究研究多种矿物对环境污染物转化的影响。在本文中,我们研究了二氯乙酰胺安全剂苯甲沙星,呋喃唑和二氯甲酰胺在含有Fe(II)修饰的赤铁矿和水钠锰矿的二元矿物厌氧系统中的反应性。随着Fe(II)与Mn(IV)氧化物的摩尔比增加,苯甲酸酯和呋喃唑的转化率增加。在取样时间(6小时)内,安全性更强的二氯mid胺没有明显转化。pH缓冲液的浓度([MOPS] = 10–50 mM),离子强度([NaCl] = 10–200 mM)和溶质的添加顺序(例如,紧随其后的是Fe(II),反之亦然)不会显着影响所检查的二氯乙酰胺安全剂在Fe(II)+赤铁矿浆料中的转化率。在含有Cr(H 2 O)6 2+(作为模型均质还原剂)的溶液中,包括除草剂S-甲草胺和三种表面活性剂在内的农用化学助剂的存在也基本不影响安全转化率。这项研究是最早研究中等复杂性实验室系统(例如,含有农药化学共制剂或矿物质相混合物的系统)在评估新兴污染物(例如二氯乙酰胺安全剂)的环境命运时。
更新日期:2020-09-22
中文翻译:

二氯乙酰胺安全剂的还原性转化:农药共制剂和氧化铁+氧化锰二元矿物系统的影响。
除草剂的毒性作用通常选择不完全,会损害农作物。安全剂是通常添加到除草剂配方中的“惰性”成分,可保护农作物免受除草剂引起的伤害。先前已证明二氯乙酰胺安全剂在厌氧的非生物系统中进行还原脱氯,该系统含有铁(氢)氧化物矿物质(针铁矿或赤铁矿),并经Fe(II)改性。锰氧化物(例如,水钠锰矿)是重要的氧化还原活性物种,经常与氧化铁(氢氧化物)同时存在,但很少有研究研究多种矿物对环境污染物转化的影响。在本文中,我们研究了二氯乙酰胺安全剂苯甲沙星,呋喃唑和二氯甲酰胺在含有Fe(II)修饰的赤铁矿和水钠锰矿的二元矿物厌氧系统中的反应性。随着Fe(II)与Mn(IV)氧化物的摩尔比增加,苯甲酸酯和呋喃唑的转化率增加。在取样时间(6小时)内,安全性更强的二氯mid胺没有明显转化。pH缓冲液的浓度([MOPS] = 10–50 mM),离子强度([NaCl] = 10–200 mM)和溶质的添加顺序(例如,紧随其后的是Fe(II),反之亦然)不会显着影响所检查的二氯乙酰胺安全剂在Fe(II)+赤铁矿浆料中的转化率。在含有Cr(H 2 O)6 2+(作为模型均质还原剂)的溶液中,包括除草剂S-甲草胺和三种表面活性剂在内的农用化学助剂的存在也基本不影响安全转化率。这项研究是最早研究中等复杂性实验室系统(例如,含有农药化学共制剂或矿物质相混合物的系统)在评估新兴污染物(例如二氯乙酰胺安全剂)的环境命运时。



























 京公网安备 11010802027423号
京公网安备 11010802027423号