当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Al(III)-NTA-fluoride: a simple model system for Al–F binding with interesting thermodynamics
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-09-22 , DOI: 10.1039/d0dt02644a Eliška Hacaperková 1, 2, 3, 4, 5 , Adam Jaroš 5, 6, 7, 8 , Jan Kotek 1, 2, 3, 4, 5 , Johannes Notni 9, 10, 11, 12 , Michal Straka 5, 6, 7, 8 , Vojtěch Kubíček 1, 2, 3, 4, 5 , Petr Hermann 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-09-22 , DOI: 10.1039/d0dt02644a Eliška Hacaperková 1, 2, 3, 4, 5 , Adam Jaroš 5, 6, 7, 8 , Jan Kotek 1, 2, 3, 4, 5 , Johannes Notni 9, 10, 11, 12 , Michal Straka 5, 6, 7, 8 , Vojtěch Kubíček 1, 2, 3, 4, 5 , Petr Hermann 1, 2, 3, 4, 5
Affiliation
|
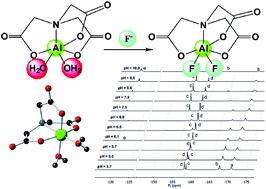
Al(III) complexes are extensively studied as [18F]fluoride carriers in positron emission tomography. However, our limited knowledge on their thermodynamic and kinetic properties has hindered efforts to easily prepare radiochemically pure compounds while simultaneously reducing the overall labeling time. Thus, to improve our understanding of fluoride binding to coordinatively unsaturated Al(III) complexes, we investigated the ternary system Al(III)–H3NTA–F− (H3NTA = nitrilo-triacetic acid) by NMR, potentiometry and X-ray diffraction. Our results show that the [Al(NTA)] complex binds two water molecules, which are replaced by fluorides. Individual species and isomers show separate 19F NMR signals and different stability constants. The two available positions on the [Al(NTA)] complex feature significantly different properties in terms of basicity of the coordinated water molecules and preferential binding of fluoride anions. Fluorides are effectively bound in weakly acidic or neutral solutions, whereas hydroxido species are preferentially formed in alkaline solutions. Our experimental observations were rationalized by theoretical calculations: predictions of the energy ordering of complexes and isomers, interpretation of 19F NMR chemical shifts, and natural bonding orbital analysis. Radiolabeling of [Al(NTA)] with [18F]fluoride gave low yields that confirmed a high affinity of the complex for hydroxide anions.
中文翻译:

Al(III)-NTA-氟化物:Al-F结合的简单模型系统,具有有趣的热力学
Al(III)配合物在正电子发射断层扫描中作为[ 18 F]氟化物载体被广泛研究。但是,我们对它们的热力学和动力学性质的了解有限,阻碍了容易地制备放射化学纯化合物同时减少总标记时间的努力。因此,为了提高我们的氟化物结合配位不饱和的Al(理解III)配合物中,我们调查的三元体系的Al(III)-H 3 NTA-F -(H 3NMR,电位法和X射线衍射分析(NTA =次氮基三乙酸)。我们的结果表明,[Al(NTA)]配合物结合了两个被氟化物取代的水分子。单个物质和异构体显示出不同的19 F NMR信号和不同的稳定性常数。在[Al(NTA)]络合物上的两个可用位置在配位水分子的碱性和氟化物阴离子的优先结合方面具有明显不同的特性。氟化物可有效地结合在弱酸性或中性溶液中,而羟基则优先在碱性溶液中形成。我们的实验观察结果通过理论计算得到了合理化:对络合物和异构体的能级预测,对19的解释F NMR化学位移和自然键轨道分析。用[ 18 F]氟化物对[Al(NTA)]进行放射性标记,收率低,证实了该配合物对氢氧根阴离子的亲和力高。
更新日期:2020-10-12
中文翻译:

Al(III)-NTA-氟化物:Al-F结合的简单模型系统,具有有趣的热力学
Al(III)配合物在正电子发射断层扫描中作为[ 18 F]氟化物载体被广泛研究。但是,我们对它们的热力学和动力学性质的了解有限,阻碍了容易地制备放射化学纯化合物同时减少总标记时间的努力。因此,为了提高我们的氟化物结合配位不饱和的Al(理解III)配合物中,我们调查的三元体系的Al(III)-H 3 NTA-F -(H 3NMR,电位法和X射线衍射分析(NTA =次氮基三乙酸)。我们的结果表明,[Al(NTA)]配合物结合了两个被氟化物取代的水分子。单个物质和异构体显示出不同的19 F NMR信号和不同的稳定性常数。在[Al(NTA)]络合物上的两个可用位置在配位水分子的碱性和氟化物阴离子的优先结合方面具有明显不同的特性。氟化物可有效地结合在弱酸性或中性溶液中,而羟基则优先在碱性溶液中形成。我们的实验观察结果通过理论计算得到了合理化:对络合物和异构体的能级预测,对19的解释F NMR化学位移和自然键轨道分析。用[ 18 F]氟化物对[Al(NTA)]进行放射性标记,收率低,证实了该配合物对氢氧根阴离子的亲和力高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号