当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct (Hetero)arylation of Heteroarenes Catalyzed by Unsymmetrical Pd-PEPPSI-NHC Complexes under Mild Conditions
Organometallics ( IF 2.8 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.organomet.0c00494 A-Xiang Song 1 , Xiao-Xiao Zeng 1 , Bei-Bei Ma 1 , Chang Xu 1 , Feng-Shou Liu 1
Organometallics ( IF 2.8 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.organomet.0c00494 A-Xiang Song 1 , Xiao-Xiao Zeng 1 , Bei-Bei Ma 1 , Chang Xu 1 , Feng-Shou Liu 1
Affiliation
|
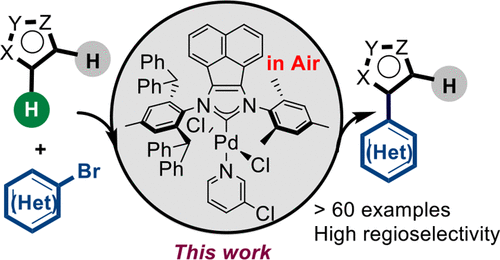
With the aim of developing a facile and efficient method to access structurally intriguing and valuable functionalized (hetero)aryls, two unsymmetrical Pd-PEPPSI-type NHC complexes (PEPPSI, pyridine-enhanced precatalyst preparation, stabilization, and initiation; NHC, N-heterocyclic carbene) were designed and synthesized to catalyze the direct arylation of heteroarenes with (hetero)aryl bromides. The results demonstrated that the utilization of this “unsymmetrical” strategy led to much higher efficiency in comparison to the commonly used C2-symmetric Pd-PEPPSI-type NHC complexes. Furthermore, a broad range of heteroaromatics and (hetero)aryl bromide partners with a wide variety of functional groups were all amenable to the developed protocol even at as low as 0.05 mol % catalyst loading and under aerobic conditions. More importantly, along with our study, we also found that the present protocol could provide expedient access to the gram-scale synthesis of the muscle relaxant drug dantrolene and conjugated mesopolymers.
中文翻译:

非对称Pd-PEPPSI-NHC配合物在温和条件下催化杂芳烃的直接(杂)芳基化
为了开发一种方便有效的方法来获得结构上引人入胜的有价值的官能化(杂)芳基,制备了两种不对称的Pd-PEPPSI型NHC配合物(PEPPSI,吡啶增强的前催化剂的制备,稳定和引发; NHC,N-杂环碳烯被设计和合成,以催化杂芳烃与(杂)芳基溴化物的直接芳基化。结果表明,与通常使用的C 2相比,这种“非对称”策略的利用导致更高的效率。对称的Pd-PEPPSI型NHC复合物。此外,即使在低至0.05 mol%的催化剂负载量和好氧条件下,具有各种官能团的各种杂芳族和(杂)芳基溴伙伴也都符合开发的方案。更重要的是,随着我们的研究,我们还发现,本协议可以方便地访问肌肉松弛药dantrolene和共轭共聚聚合物的克级合成方法。
更新日期:2020-10-12
中文翻译:

非对称Pd-PEPPSI-NHC配合物在温和条件下催化杂芳烃的直接(杂)芳基化
为了开发一种方便有效的方法来获得结构上引人入胜的有价值的官能化(杂)芳基,制备了两种不对称的Pd-PEPPSI型NHC配合物(PEPPSI,吡啶增强的前催化剂的制备,稳定和引发; NHC,N-杂环碳烯被设计和合成,以催化杂芳烃与(杂)芳基溴化物的直接芳基化。结果表明,与通常使用的C 2相比,这种“非对称”策略的利用导致更高的效率。对称的Pd-PEPPSI型NHC复合物。此外,即使在低至0.05 mol%的催化剂负载量和好氧条件下,具有各种官能团的各种杂芳族和(杂)芳基溴伙伴也都符合开发的方案。更重要的是,随着我们的研究,我们还发现,本协议可以方便地访问肌肉松弛药dantrolene和共轭共聚聚合物的克级合成方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号