当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Manufacturing Processes for the Carboxylic Acid Key Intermediate of Lusutrombopag: One-Pot Reaction Process of Formylation and the Horner–Wadsworth–Emmons Reaction
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.oprd.0c00311 Takaharu Matsuura 1 , Yusuke Sato 2 , Yutaka Nishino 2 , Tadafumi Komurasaki 3 , Yoshiaki Imamura 4 , Makoto Kakinuma 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-09-22 , DOI: 10.1021/acs.oprd.0c00311 Takaharu Matsuura 1 , Yusuke Sato 2 , Yutaka Nishino 2 , Tadafumi Komurasaki 3 , Yoshiaki Imamura 4 , Makoto Kakinuma 2
Affiliation
|
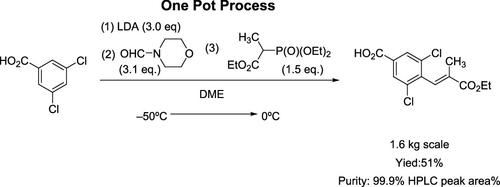
We describe the development of a one-pot preparation process of (E)-3,5-dichloro-4-(3-ethoxy-2-methyl-3-oxoprop-1-en-1-yl)benzoic acid (3), which is a key carboxylic acid intermediate of lusutrombopag. This one-pot reaction process is composed of lithiation of 3,5-dichlorobenzoic acid with lithium diisopropylamide (LDA), formylation using N-formylmorpholine, followed by olefination employing the Horner–Wadsworth–Emmons reaction with triethyl 2-phosphonopropionate. This method enabled kilogram-scale manufacturing of carboxylic acid 3, a key intermediate of lusutrombopag with high purity, together with a reduced number of steps, improvement of yield, and avoidance of a cumbersome procedure for isolation of the intermediate.
中文翻译:

Lusutrombopag羧酸关键中间体的生产工艺开发:甲酰化的一锅反应过程和霍纳-沃兹沃思-埃蒙斯反应
我们描述了(E)-3,5-二氯-4-(3-乙氧基-2-甲基-3-氧代丙-1-烯-1-基)苯甲酸的一锅法制备工艺的发展(3) ,它是鲁舒莫巴的关键羧酸中间体。一锅法反应过程包括3,5-二氯苯甲酸与二异丙基氨基化锂(LDA)的锂化,使用N-甲酰基吗啉的甲酰化,然后使用Horner-Wadsworth-Emmons反应与2-膦酸丙酸三乙酯的烯化。该方法使公斤级规模的羧酸3(鲁舒通帕的关键中间体)具有高纯度,并减少了步骤数,提高了收率,并且避免了繁琐的中间体分离步骤。
更新日期:2020-11-21
中文翻译:

Lusutrombopag羧酸关键中间体的生产工艺开发:甲酰化的一锅反应过程和霍纳-沃兹沃思-埃蒙斯反应
我们描述了(E)-3,5-二氯-4-(3-乙氧基-2-甲基-3-氧代丙-1-烯-1-基)苯甲酸的一锅法制备工艺的发展(3) ,它是鲁舒莫巴的关键羧酸中间体。一锅法反应过程包括3,5-二氯苯甲酸与二异丙基氨基化锂(LDA)的锂化,使用N-甲酰基吗啉的甲酰化,然后使用Horner-Wadsworth-Emmons反应与2-膦酸丙酸三乙酯的烯化。该方法使公斤级规模的羧酸3(鲁舒通帕的关键中间体)具有高纯度,并减少了步骤数,提高了收率,并且避免了繁琐的中间体分离步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号