当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Measurement and Thermodynamic Modeling of Capecitabine (an Anticancer Drug) Solubility in Supercritical Carbon Dioxide in a Ternary System: Effect of Different Cosolvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.jced.0c00183 Nedasadat Saadati Ardestani 1 , Navid Yeganeh Majd 2 , Mitra Amani 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.jced.0c00183 Nedasadat Saadati Ardestani 1 , Navid Yeganeh Majd 2 , Mitra Amani 2
Affiliation
|
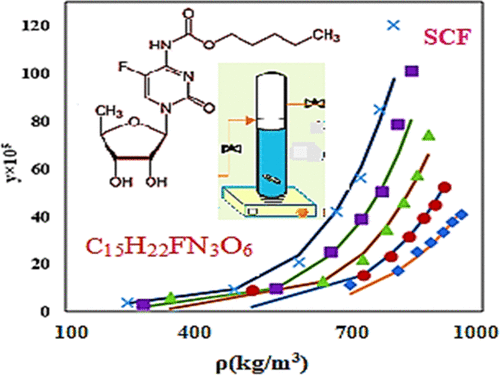
Solubility of capecitabine (CPT) in a ternary system (supercritical carbon dioxide (sc-CO2) + CPT + [methanol, ethanol, and dimethyl sulfoxide (DMSO)] as cosolvents) was measured at different temperatures (308.15–348.15 K) and pressures (100–350 bar). CPT solubility in the presence of methanol, ethanol, and DMSO was in the range of 3.18 × 10–5 to 120.29 × 10–5, 0.64 × 10–5 to 71.9 × 10–5, and 0.85 × 10–5 to 94.8 × 10–5 mole fraction, respectively. A significant solubility improvement of CPT in sc-CO2 is shown by comparison with its solubility in a binary system (sc-CO2 + CPT) reported in the literature (Yamini et al. 2012). The solubility data were correlated by several well-known empirical models of ternary systems. Among them, the Jouyban et al. model produced the best correlation with average absolute relative deviation (AARD %) of 10.58, 9.21, and 8.92% for methanol, ethanol, and DMSO, respectively. Also, the solubility data of the methanol cosolvent were correlated by Peng–Robinson (PR-vdW2), Soave–Redlich–Kowng (SRK-vdW2), and perturbed-chain polar statistical associating fluid theory (PCP-SAFT) thermodynamic equation of state-based models. According to the mean AARD % value of PR equation of state (EoS) (20.32%) and corresponding Radj (0.9866), it could be concluded that PR EoS shows a better performance in comparison with other thermodynamic models.
中文翻译:

卡培他滨(一种抗癌药物)在三元体系中超临界二氧化碳中的溶解度的实验测量和热力学模型:不同助溶剂的影响
卡培他滨(CPT)在三元体系(超临界二氧化碳(sc-CO 2)+ CPT + [甲醇,乙醇和二甲基亚砜(DMSO)]作为助溶剂)中的溶解度在不同温度(308.15–348.15 K)和压力(100–350 bar)。在甲醇,乙醇和DMSO存在下,CPT的溶解度范围为3.18×10–5至120.29×10–5、0.64×10–5至71.9×10–5和0.85×10–5至94.8× 10–5摩尔分数。与文献中报道的CPT在二元体系(sc-CO 2 + CPT)中的溶解度进行比较,可以看出CPT在sc-CO 2中的溶解度有了显着提高(Yamini et al 。2012)。溶解度数据通过几种众所周知的三元系统的经验模型进行关联。其中,Jouyban等人。模型产生的最佳相关性是甲醇,乙醇和DMSO的平均绝对相对偏差(AARD%)分别为10.58、9.21和8.92%。另外,甲醇助溶剂的溶解度数据与Peng-Robinson(PR-vdW2),Soave-Redlich-Kowng(SRK-vdW2)和扰动链极性统计缔合流体理论(PCP-SAFT)热力学状态方程相关。基于模型。根据PR状态方程(EoS)(20.32%)的平均AARD%值和相应的R adj(0.9866),可以得出结论PR EoS与其他热力学模型相比具有更好的性能。
更新日期:2020-10-08
中文翻译:

卡培他滨(一种抗癌药物)在三元体系中超临界二氧化碳中的溶解度的实验测量和热力学模型:不同助溶剂的影响
卡培他滨(CPT)在三元体系(超临界二氧化碳(sc-CO 2)+ CPT + [甲醇,乙醇和二甲基亚砜(DMSO)]作为助溶剂)中的溶解度在不同温度(308.15–348.15 K)和压力(100–350 bar)。在甲醇,乙醇和DMSO存在下,CPT的溶解度范围为3.18×10–5至120.29×10–5、0.64×10–5至71.9×10–5和0.85×10–5至94.8× 10–5摩尔分数。与文献中报道的CPT在二元体系(sc-CO 2 + CPT)中的溶解度进行比较,可以看出CPT在sc-CO 2中的溶解度有了显着提高(Yamini et al 。2012)。溶解度数据通过几种众所周知的三元系统的经验模型进行关联。其中,Jouyban等人。模型产生的最佳相关性是甲醇,乙醇和DMSO的平均绝对相对偏差(AARD%)分别为10.58、9.21和8.92%。另外,甲醇助溶剂的溶解度数据与Peng-Robinson(PR-vdW2),Soave-Redlich-Kowng(SRK-vdW2)和扰动链极性统计缔合流体理论(PCP-SAFT)热力学状态方程相关。基于模型。根据PR状态方程(EoS)(20.32%)的平均AARD%值和相应的R adj(0.9866),可以得出结论PR EoS与其他热力学模型相比具有更好的性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号