Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Route to the Synthesis of Benzamide-Based 5-Aminopyrazoles and Their Fused Heterocycles Showing Remarkable Antiavian Influenza Virus Activity
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-21 , DOI: 10.1021/acsomega.0c02675 Ali M. S. Hebishy 1 , Hagar T. Salama 1 , Galal H. Elgemeie 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-21 , DOI: 10.1021/acsomega.0c02675 Ali M. S. Hebishy 1 , Hagar T. Salama 1 , Galal H. Elgemeie 1
Affiliation
|
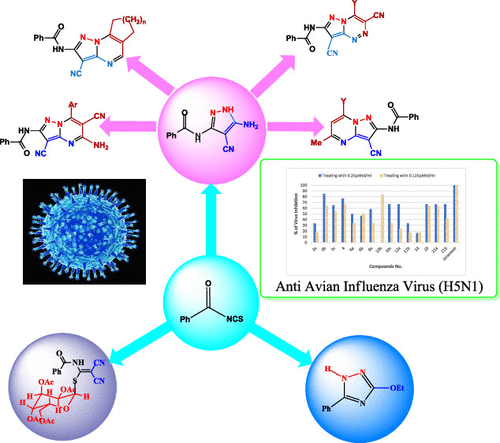
This study describes a new route to the synthesis of novel benzamide-based 5-aminopyrazoles and their corresponding pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives. Benzamide-based 5-aminopyrazoles were prepared through a reaction of benzoyl isothiocyanate with malononitrile in KOH–EtOH followed by alkylation with alkyl halides and then a reaction with hydrazine. In an attempt to react benzoyl isothiocyanate with ethyl cyanoacetate in KOH–EtOH followed by alkylation with methyl iodide at room temperature and then a reaction with hydrazine has resulted in the formation of 3-ethoxy-5-phenyl-1H-1,2,4-triazole. The structures of the new compounds were characterized by mass spectroscopy, 1H nuclear magnetic resonance (1H NMR) spectroscopy, infrared spectroscopy (IR), and X-ray analysis. The new compounds were tested in vitro for their anti-influenza A virus (subtype H5N1) activity. Among the synthesized compounds, eight compounds 3b, 4, 10b, 10c, 12a, 19, 21a, and 21b were found to possess significant antiviral activities against bird flu influenza (H5N1) with viral reduction in the range of 85–65%.
中文翻译:

苯甲酰胺基5-氨基吡唑及其融合杂环显示出显着的抗禽流感病毒活性的新途径
这项研究描述了一种新的合成基于苯甲酰胺的5-氨基吡唑及其相应的吡唑并[1,5- a ]嘧啶和吡唑并[5,1- c ] [1,2,4]三嗪衍生物的新途径。苯甲酰胺基的5-氨基吡唑是通过使异硫氰酸苯甲酰与丙二腈在KOH-EtOH中反应,然后与烷基卤化物进行烷基化,然后与肼反应来制备的。为了使异硫氰酸苯甲酰基酯与氰基乙酸乙酯在KOH-EtOH中反应,然后在室温下与甲基碘烷基化,然后与肼反应,导致生成了3-乙氧基-5-苯基-1 H -1,2, 4-三唑。新化合物的结构通过质谱表征,1H核磁共振(1 H NMR)光谱,红外光谱(IR)和X射线分析。在体外测试了这些新化合物的抗甲型流感病毒(H5N1亚型)活性。中合成的化合物,八种化合物3b的,4,10B,10C,12A,19,21A,和21B被发现具有抗禽流感甲型(H5N1)具有在85〜65%的范围内病毒减少显著的抗病毒活性。
更新日期:2020-10-06
中文翻译:

苯甲酰胺基5-氨基吡唑及其融合杂环显示出显着的抗禽流感病毒活性的新途径
这项研究描述了一种新的合成基于苯甲酰胺的5-氨基吡唑及其相应的吡唑并[1,5- a ]嘧啶和吡唑并[5,1- c ] [1,2,4]三嗪衍生物的新途径。苯甲酰胺基的5-氨基吡唑是通过使异硫氰酸苯甲酰与丙二腈在KOH-EtOH中反应,然后与烷基卤化物进行烷基化,然后与肼反应来制备的。为了使异硫氰酸苯甲酰基酯与氰基乙酸乙酯在KOH-EtOH中反应,然后在室温下与甲基碘烷基化,然后与肼反应,导致生成了3-乙氧基-5-苯基-1 H -1,2, 4-三唑。新化合物的结构通过质谱表征,1H核磁共振(1 H NMR)光谱,红外光谱(IR)和X射线分析。在体外测试了这些新化合物的抗甲型流感病毒(H5N1亚型)活性。中合成的化合物,八种化合物3b的,4,10B,10C,12A,19,21A,和21B被发现具有抗禽流感甲型(H5N1)具有在85〜65%的范围内病毒减少显著的抗病毒活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号