当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unconventional Macrocyclizations in Natural Product Synthesis
ACS Central Science ( IF 12.7 ) Pub Date : 2020-09-21 , DOI: 10.1021/acscentsci.0c00599 Iakovos Saridakis 1 , Daniel Kaiser 1 , Nuno Maulide 1, 2
ACS Central Science ( IF 12.7 ) Pub Date : 2020-09-21 , DOI: 10.1021/acscentsci.0c00599 Iakovos Saridakis 1 , Daniel Kaiser 1 , Nuno Maulide 1, 2
Affiliation
|
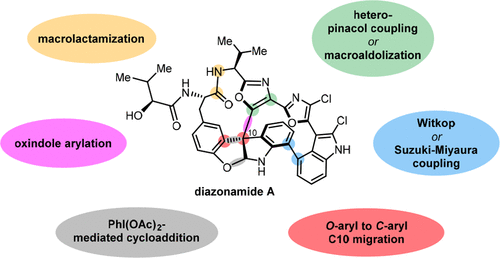
Over the past several decades, macrocyclic compounds have emerged as increasingly significant therapeutic candidates in drug discovery. Their pharmacological activity hinges on their rotationally restricted three-dimensional orientation, resulting in a unique conformational preorganization and a high enthalpic gain as a consequence of high-affinity macrocycle–protein binding interactions. Synthetic access to macrocyclic drug candidates is therefore crucial. From a synthetic point of view, the efficiency of macrocyclization events commonly suffers from entropic penalties as well as undesired intermolecular couplings (oligomerization). Although over the past several decades ring-closing metathesis, macrolactonization, or macrolactamization have become strategies of choice, the toolbox of organic synthesis provides a great number of versatile transformations beyond the aforementioned. This Outlook focuses on a selection of examples employing what we term unconventional macrocyclizations toward the synthesis of natural products or analogues.
中文翻译:

天然产物合成中的非常规大环化
在过去的几十年中,大环化合物已成为药物发现中越来越重要的治疗候选物。它们的药理活性取决于其旋转受限的三维方向,由于高亲和力的大环-蛋白质结合相互作用,导致独特的构象预组织和高焓增加。因此,合成获得大环候选药物至关重要。从综合的角度来看,大环化事件的效率通常遭受熵的损失以及不希望的分子间偶联(低聚)。尽管在过去的几十年中,闭环复分解,大环内酯化或大环内酰胺化已成为首选策略,除了上述功能,有机合成工具箱还提供了许多通用的转换功能。本展望着眼于采用我们所称的例子合成天然产物或类似物的非常规大环化。
更新日期:2020-11-25
中文翻译:

天然产物合成中的非常规大环化
在过去的几十年中,大环化合物已成为药物发现中越来越重要的治疗候选物。它们的药理活性取决于其旋转受限的三维方向,由于高亲和力的大环-蛋白质结合相互作用,导致独特的构象预组织和高焓增加。因此,合成获得大环候选药物至关重要。从综合的角度来看,大环化事件的效率通常遭受熵的损失以及不希望的分子间偶联(低聚)。尽管在过去的几十年中,闭环复分解,大环内酯化或大环内酰胺化已成为首选策略,除了上述功能,有机合成工具箱还提供了许多通用的转换功能。本展望着眼于采用我们所称的例子合成天然产物或类似物的非常规大环化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号