Structure ( IF 4.4 ) Pub Date : 2020-09-22 , DOI: 10.1016/j.str.2020.09.001 Lucien Fabre 1 , Abigail T Ntreh 2 , Amira Yazidi 3 , Inga V Leus 2 , Jon W Weeks 2 , Sudipta Bhattacharyya 4 , Jakob Ruickoldt 5 , Isabelle Rouiller 6 , Helen I Zgurskaya 2 , Jurgen Sygusch 3
|
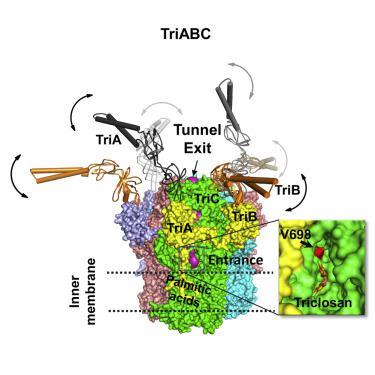
The structure of the TriABC inner membrane component of the triclosan/SDS-specific efflux pump from Pseudomonas aeruginosa was determined by cryoelectron microscopy to 4.5 Å resolution. The complete structure of the inner membrane transporter TriC of the resistance-nodulation-division (RND) superfamily was solved, including a partial structure of the fused periplasmic membrane fusion subunits, TriA and TriB. The substrate-free conformation of TriABC represents an intermediate step in efflux complex assembly before the engagement of the outer membrane channel. Structural analysis identified a tunnel network whose constriction impedes substrate efflux, indicating inhibition of TriABC in the unengaged state. Blind docking studies revealed binding to TriC at the same loci by substrates and bulkier non-substrates. Together with functional analyses, we propose that selective substrate translocation involves conformational gating at the tunnel narrowing that, together with conformational ordering of TriA and TriB, creates an engaged state capable of mediating substrate efflux.
中文翻译:

来自铜绿假单胞菌的 TriABC 三氯生外排泵的“药物清除”状态
铜绿假单胞菌三氯生/SDS特异性外排泵TriABC内膜组分的结构通过冷冻电子显微镜测定到 4.5 Å 分辨率。解决了抗结节分裂 (RND) 超家族内膜转运蛋白 TriC 的完整结构,包括融合周质膜融合亚基 TriA 和 TriB 的部分结构。TriABC 的无底物构象代表了在外膜通道接合之前外排复合体组装的中间步骤。结构分析确定了一个隧道网络,其收缩阻碍了底物流出,表明 TriABC 在未接合状态下受到抑制。盲对接研究显示底物和较大的非底物在相同位点与 TriC 结合。结合功能分析,我们提出选择性底物易位涉及隧道变窄处的构象门控,











































 京公网安备 11010802027423号
京公网安备 11010802027423号